Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The three lines on the graph are the same gas (both type and amount) for different temperatures. I will refer to them as either

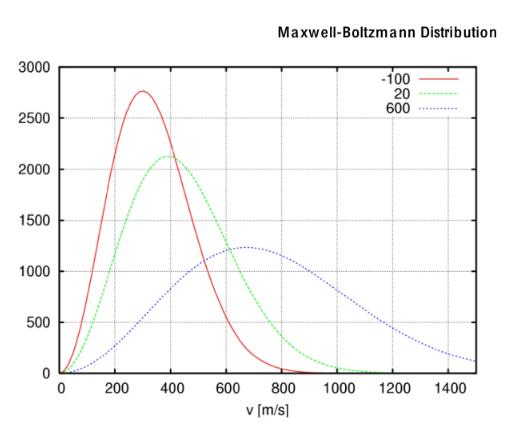

The three lines on the graph are the same gas (both type and amount) for different temperatures. I will refer to them as either solid, (-100), dashed (20) and dotted (600). The Y-axis is the number of molecules that have a given speed. 1) Why is speed equivalent to temperature? 2) Do all molecules at the same temperature... a. have the same speed? Y/N b. have the same average speed? Y/N 3000 Maxwell-Boltzmann Distribution 2500 2000 1500 1000 500 -100 20 600 0 200 400 600 800 1000 1200 1400 v [m/s] 3) State three differences in the size/shape of the curve for higher temperatures. 4) How many molecules have the following speeds: Speed Number of gas particles (SOLID LINE) Number of gas particles (DASHED LINE) Number of gas particles (DOTTED LINE) 200 400 600 = 5) Will the area under the solid line the area under the dashed line? Will either equal the area under the dotted line? 6) Draw slanted lines in the area that corresponds to all the molecules that have speeds of 600 m/s or greater for the solid line graph. 7) Using a different kind of shading (slanted lines the other direction, etc.), show all the molecules that have speeds of 600 m/s or great for the dashed lines and dotted lines. 8) Which temperature has the most molecules with speeds above 600 m/s?
Step by Step Solution
★★★★★
3.32 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


