Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The van der Waal's equation of state is given as P=vbRTv2a For sulfur dioxide at temperature, T, of 300K and a pressure, P, of 1atm,
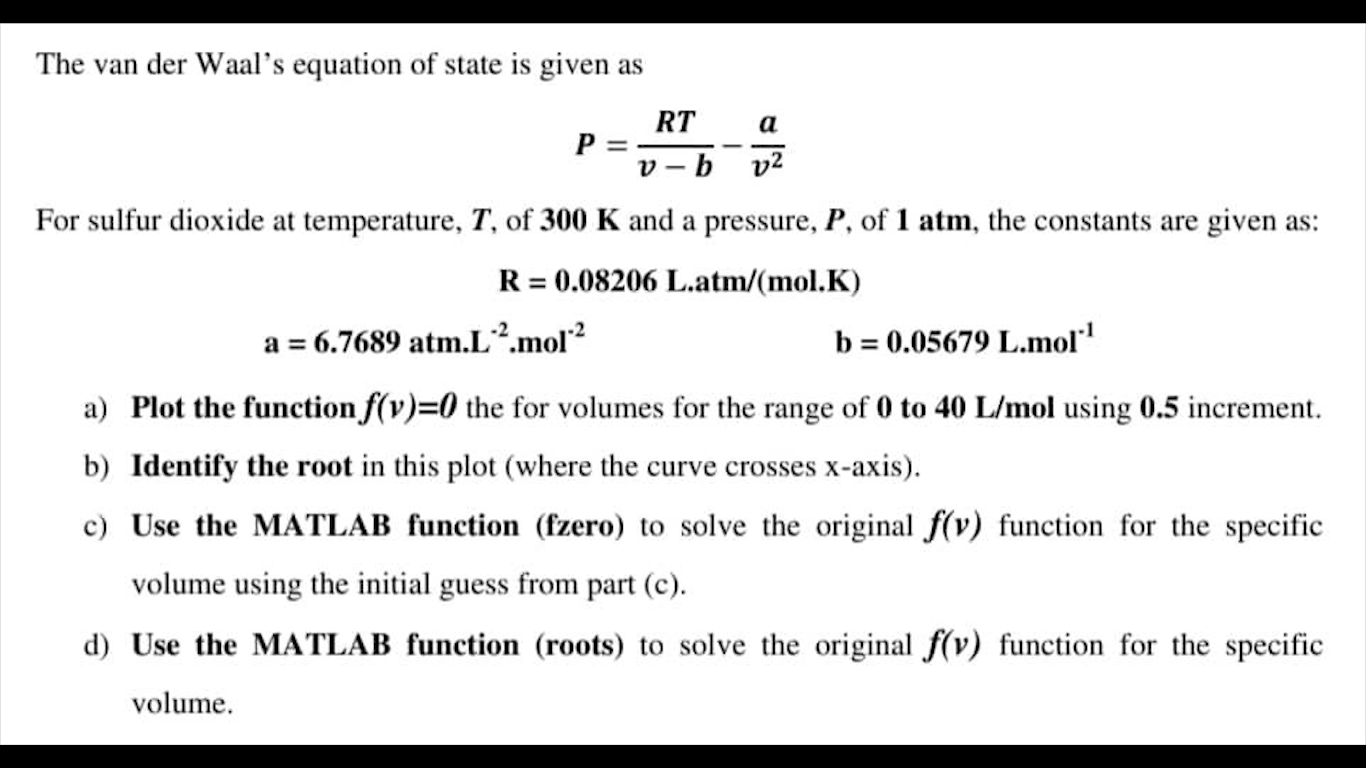 The van der Waal's equation of state is given as P=vbRTv2a For sulfur dioxide at temperature, T, of 300K and a pressure, P, of 1atm, the constants are given as: R=0.08206Latm/(mol.K)a=6.7689atmL2mol2b=0.05679Lmol1 a) Plot the function f(v)=0 the for volumes for the range of 0 to 40L/mol using 0.5 increment. b) Identify the root in this plot (where the curve crosses x-axis). c) Use the MATLAB function (fzero) to solve the original f(v) function for the specific volume using the initial guess from part (c). d) Use the MATLAB function (roots) to solve the original f(v) function for the specific volume
The van der Waal's equation of state is given as P=vbRTv2a For sulfur dioxide at temperature, T, of 300K and a pressure, P, of 1atm, the constants are given as: R=0.08206Latm/(mol.K)a=6.7689atmL2mol2b=0.05679Lmol1 a) Plot the function f(v)=0 the for volumes for the range of 0 to 40L/mol using 0.5 increment. b) Identify the root in this plot (where the curve crosses x-axis). c) Use the MATLAB function (fzero) to solve the original f(v) function for the specific volume using the initial guess from part (c). d) Use the MATLAB function (roots) to solve the original f(v) function for the specific volume Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


