Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The vapor is in equilibrium with a solution ofM and N at 85C and the solution can be assumed to be essentially ideally dilute.
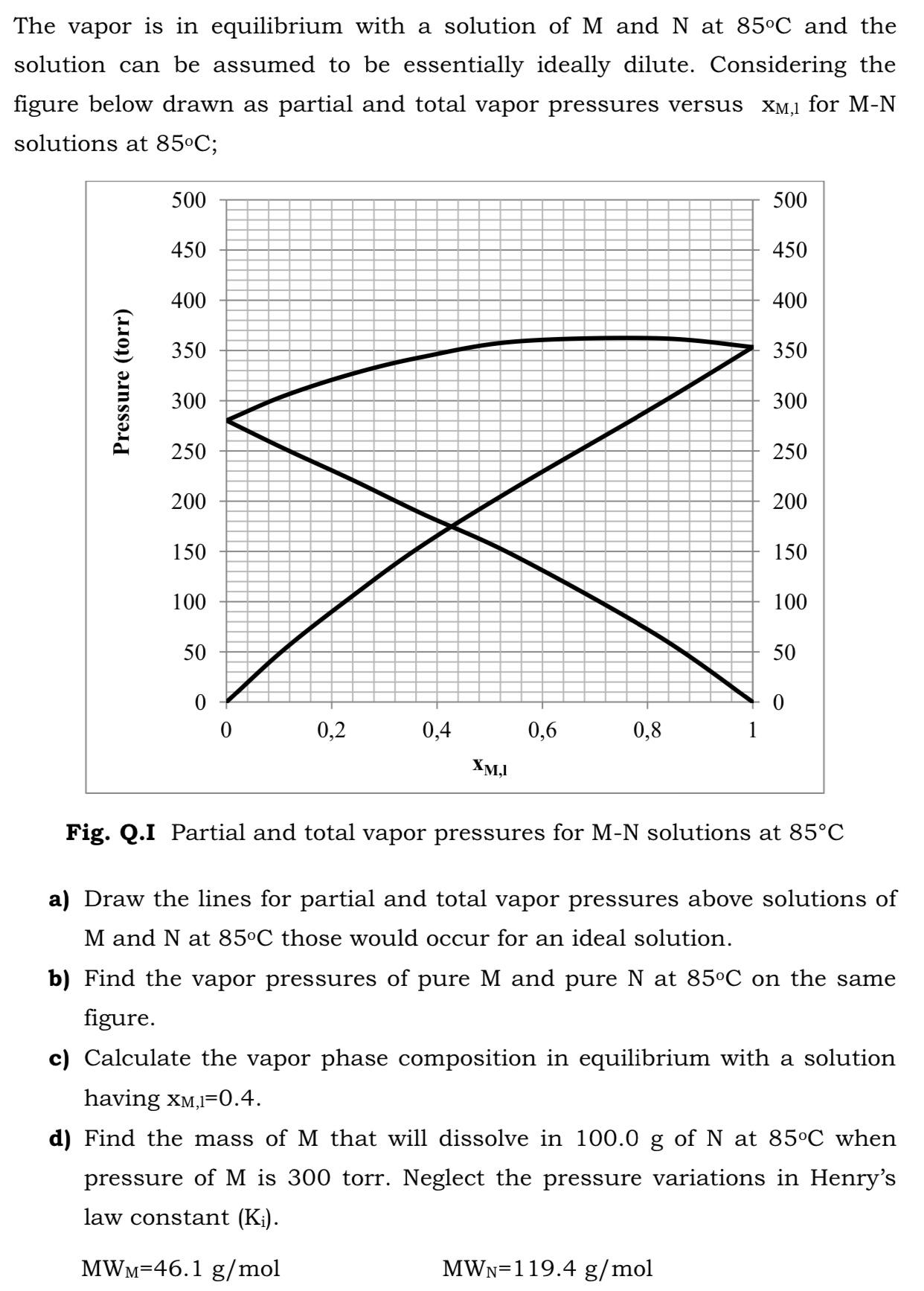
The vapor is in equilibrium with a solution ofM and N at 85C and the solution can be assumed to be essentially ideally dilute. Considering the figure below drawn as partial and total vapor pressures versus XM,1 for M-N solutions at 85C; 500 500 450 450 400 400 350 350 300 300 250 250 200 200 150 150 100 100 50 50 0,2 0,4 0,6 0,8 XM,I Fig. Q.I Partial and total vapor pressures for M-N solutions at 85C a) Draw the lines for partial and total vapor pressures above solutions of M and N at 85C those would occur for an ideal solution. b) Find the vapor pressures of pure M and pure N at 85C on the same figure. c) Calculate the vapor phase composition in equilibrium with a solution having xM,I=0.4. d) Find the mass of M that will dissolve in 100.0 g of N at 85C when pressure of M is 300 torr. Neglect the pressure variations in Henry's law constant (K). MWM=46.1 g/mol MWN=119.4 g/mol Pressure (torr)
Step by Step Solution
★★★★★
3.48 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
a The experimental and Idea l partial vapour pressures of Solu...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
636143e84e00e_235500.pdf
180 KBs PDF File
636143e84e00e_235500.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


