Question
The variation of the atomic properties Purpose; To use the data collection, to build graphs of the variation of a property against the atomic number.
The variation of the atomic properties
Purpose;
To use the data collection, to build graphs of the variation of a property against the atomic number.
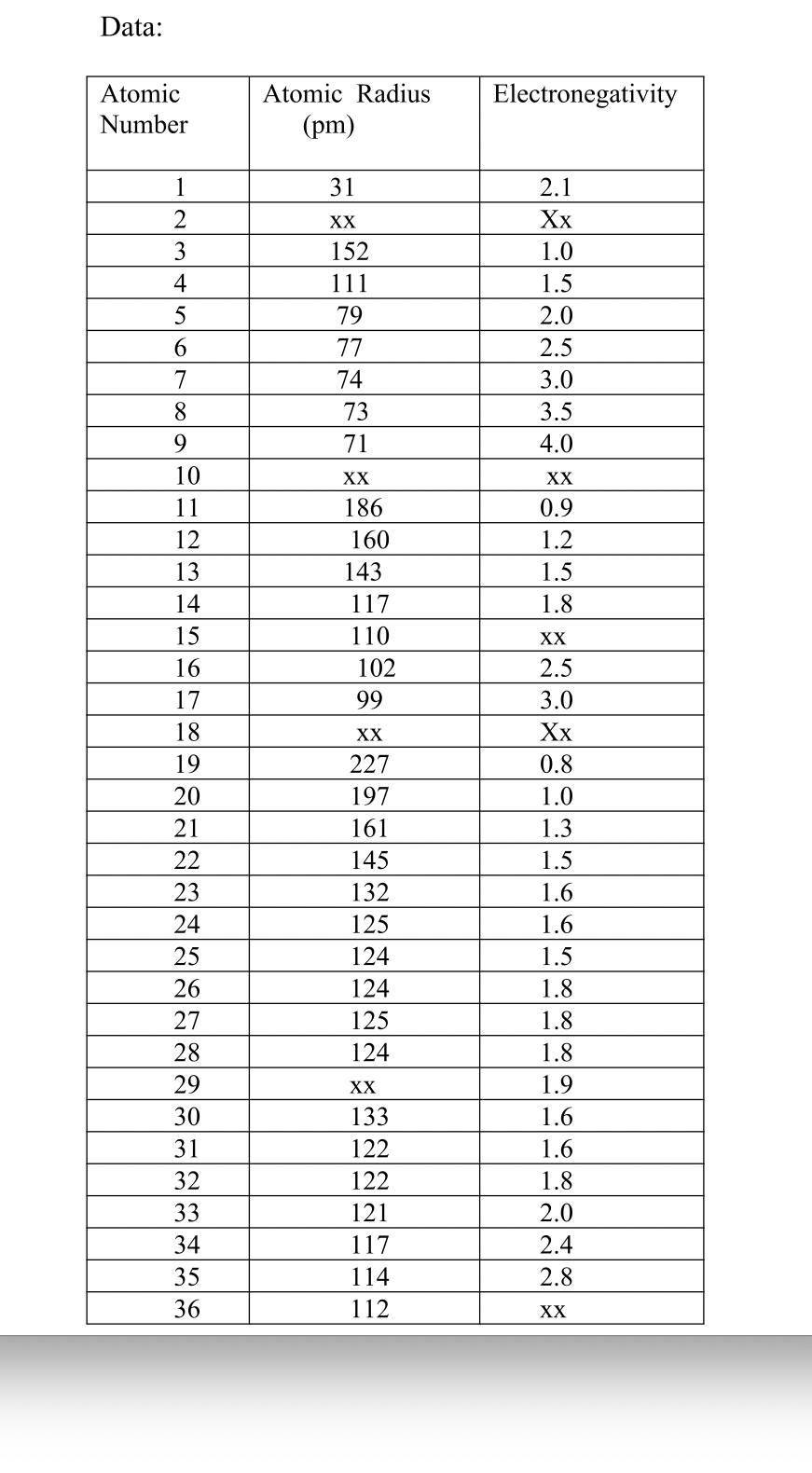
1) Graphing atomic radius
In this exercise you will plot a graph of atomic radius against the atomic number of the elements. You will examine the graph to find out whether there are any regular variations in the property.
Using the data given in the Table(next page) plot the graph for the atomic radius against the atomic number. Join consecutive points with solid straight lines. When datum for an element is missing, join the points by a broken straight line
2) Graphing Electronegativity.
You will plot a graph of electronegativity against atomic number. Using electronegativity values in the periodic table, plot a point graph of electronegativity against the atomic number for the first 36 elements. Draw the graph line.
Part B
2. What do you estimate to be the missing value of the electronegativity for element #15? Show on the graph how did you find the missing value
Electronegativity _____________
3. Using a periodic table, select an appropriate element that satisfies the condition given
particle with the larger radius : Ca2+ ion or Mg2+ ion. ____________
halogen with the highest IE. _____________
element with the highest EA in Gr16: ________________
As you go from left to write across a period, the electron affinity (decreases/ increases). ____________________
Arrange the following elements in order of increasing the atomic radius.
Sc, Ga, Co _____________________
Arrange the following elements in order of increasing reactivity.
S, Te, O, _______________________
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


