Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The voluman of a PFR (vertical cylinder) was determined for a first order isothermal gas phase reaction based on an incorrect stoichiometry. To achieve a
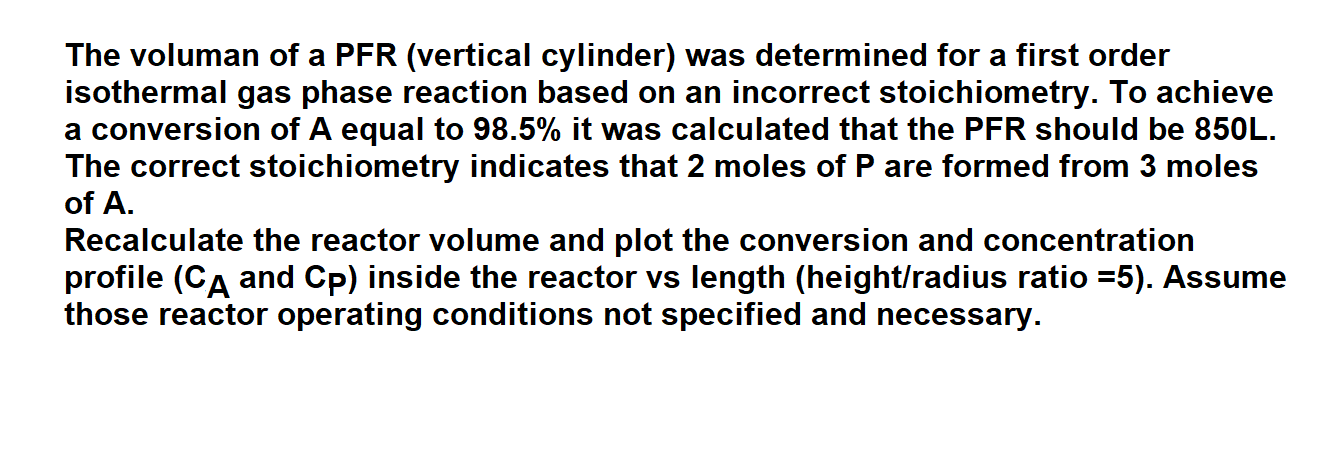 The voluman of a PFR (vertical cylinder) was determined for a first order isothermal gas phase reaction based on an incorrect stoichiometry. To achieve a conversion of A equal to 98.5% it was calculated that the PFR should be 850L. The correct stoichiometry indicates that 2 moles of P are formed from 3 moles of A. Recalculate the reactor volume and plot the conversion and concentration profile (CA and CP) inside the reactor vs length (height/radius ratio =5 ). Assume those reactor operating conditions not specified and necessary. The voluman of a PFR (vertical cylinder) was determined for a first order isothermal gas phase reaction based on an incorrect stoichiometry. To achieve a conversion of A equal to 98.5% it was calculated that the PFR should be 850L. The correct stoichiometry indicates that 2 moles of P are formed from 3 moles of A. Recalculate the reactor volume and plot the conversion and concentration profile (CA and CP) inside the reactor vs length (height/radius ratio =5 ). Assume those reactor operating conditions not specified and necessary
The voluman of a PFR (vertical cylinder) was determined for a first order isothermal gas phase reaction based on an incorrect stoichiometry. To achieve a conversion of A equal to 98.5% it was calculated that the PFR should be 850L. The correct stoichiometry indicates that 2 moles of P are formed from 3 moles of A. Recalculate the reactor volume and plot the conversion and concentration profile (CA and CP) inside the reactor vs length (height/radius ratio =5 ). Assume those reactor operating conditions not specified and necessary. The voluman of a PFR (vertical cylinder) was determined for a first order isothermal gas phase reaction based on an incorrect stoichiometry. To achieve a conversion of A equal to 98.5% it was calculated that the PFR should be 850L. The correct stoichiometry indicates that 2 moles of P are formed from 3 moles of A. Recalculate the reactor volume and plot the conversion and concentration profile (CA and CP) inside the reactor vs length (height/radius ratio =5 ). Assume those reactor operating conditions not specified and necessary Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


