Answered step by step
Verified Expert Solution
Question
1 Approved Answer
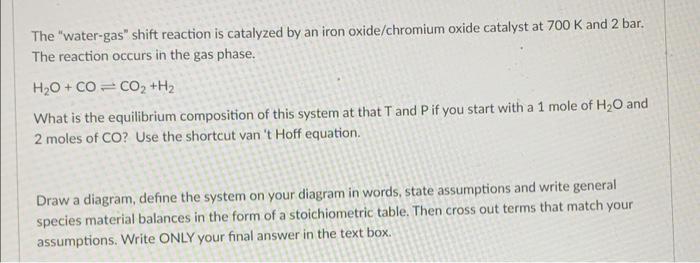
The water-gas shift reaction is catalyzed by an iron oxide/chromium oxide catalyst at 700 K and 2 bar. The reaction occurs in the gas phase.
The "water-gas" shift reaction is catalyzed by an iron oxide/chromium oxide catalyst at 700 K and 2 bar. The reaction occurs in the gas phase. H,O+CO= CO, +H2 What is the equilibrium composition of this system at that T and P if you start with a 1 mole of HO and 2 moles of CO? Use the shortcut van 't Hoff equation. Draw a diagram, define the system on your diagram in words, state assumptions and write general species material balances in the form of a stoichiometric table. Then cross out terms that match your assumptions. Write ONLY your final answer in the text box.
DRAW DIAGRAM TOO PLEASE
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


