Answered step by step
Verified Expert Solution
Question
1 Approved Answer
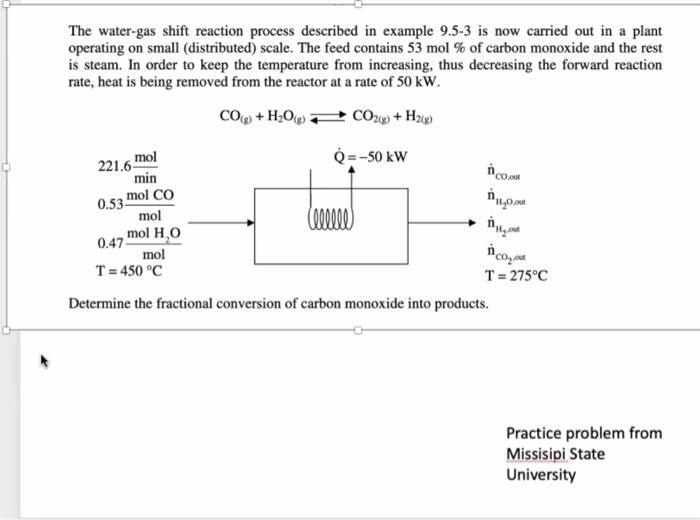
The water-gas shift reaction process described in example 9.5-3 is now carried out in a plant operating on small (distributed) scale. The feed contains 53
The water-gas shift reaction process described in example 9.5-3 is now carried out in a plant operating on small (distributed) scale. The feed contains 53 mol % of carbon monoxide and the rest is steam. In order to keep the temperature from increasing, thus decreasing the forward reaction rate, heat is being removed from the reactor at a rate of 50 kW. CO(g) + HO(g) CO2(g) + H2(g) mol min mol CO 221.6- 0.53- mol mol HO 0.47- mol T = 450 C Q=-50 kW Cllllll ico,out H0,out #11/20 Determine the fractional conversion of carbon monoxide into products. out ico. out T = 275C Practice problem from Missisipi State University
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


