Question
ther information available: The molecule does not react with bromine in chloroform but can be brominated using bromine and FeBr3, and three different monobromo products
ther information available: The molecule does not react with bromine in chloroform but can be brominated using bromine and FeBr3, and three different monobromo products are obtained.
The number of Hs in the compound have to be a multiple of what integer?
The number of Cs in the compound have to be a multiple of what integer? What is the maximum number of Cs that can be in the compound? Explain how you determined the maximum number of Cs in the compound
What functional groups are present in the compound (alkyl, aryl, ketone, etc.)? Explain your answer by referring to the information provided above.
Given your answers to a-c above write a molecular formula consistent with all the data presented.
Draw a structure for the unknown compound that is consistent with the data above
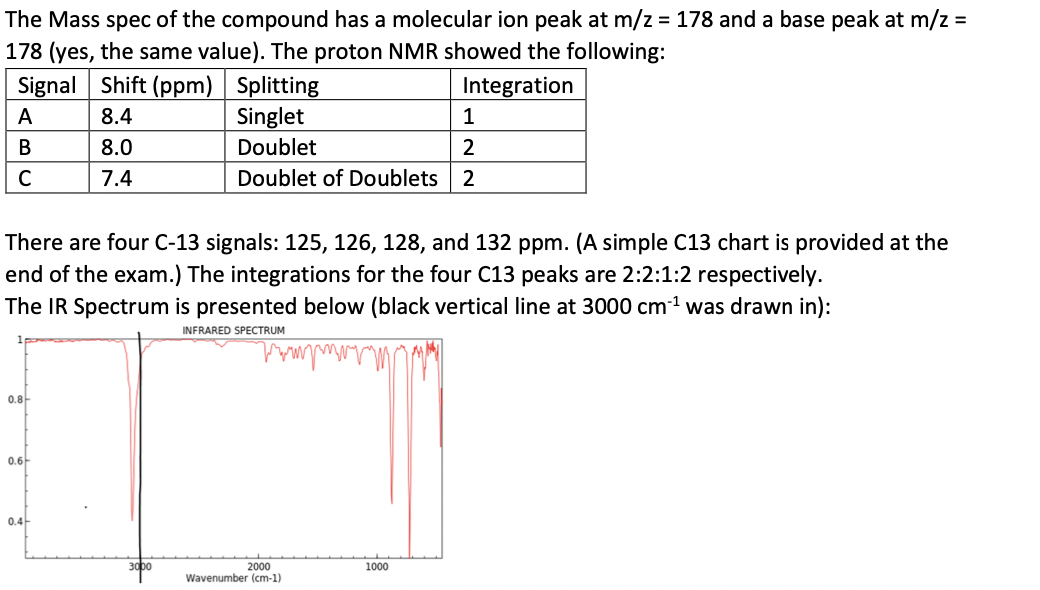
The Mass spec of the compound has a molecular ion peak at m/z=178 and a base peak at m/z= 178 (yes, the same value). The proton NMR showed the following: There are four C-13 signals: 125,126,128, and 132ppm. (A simple C13 chart is provided at the end of the exam.) The integrations for the four C13 peaks are 2:2:1:2 respectively. The IR Spectrum is presented below (black vertical line at 3000cm1 was drawn in)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


