Answered step by step
Verified Expert Solution
Question
1 Approved Answer
there are 2 questions please help. 20. A compound is composed of only C and H. It contains 88.82%C by mass and has a molar
there are 2 questions please help. 
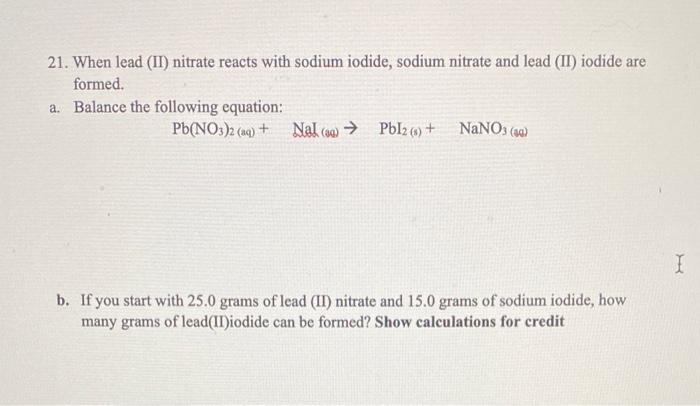

20. A compound is composed of only C and H. It contains 88.82%C by mass and has a molar mass of 54.09g/mol. What is its empirical formula? Show calculations for credit 21. When lead (II) nitrate reacts with sodium iodide, sodium nitrate and lead (II) iodide are formed. a. Balance the following equation: Pb(NO3)2(aq)+NaI()PbI2(3)+NaNO3() b. If you start with 25.0 grams of lead (II) nitrate and 15.0 grams of sodium iodide, how many grams of lead(II)iodide can be formed? Show calculations for credit c. What is the limiting reagent in the reaction? d. How much of the nonlimiting reagent or excess reagent will be left over from the reaction? Show calculations for credit e. If 6 grams of lead(II)iodide are formed in the reaction described in problem, what is the percent yield of this reaction? Show calculations for credit 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


