Answered step by step
Verified Expert Solution
Question
1 Approved Answer
There are 6 different problems. Answer all please. thats the only info given A sample of krypton gas at a pressure of 957mmHg and a
There are 6 different problems. Answer all please. 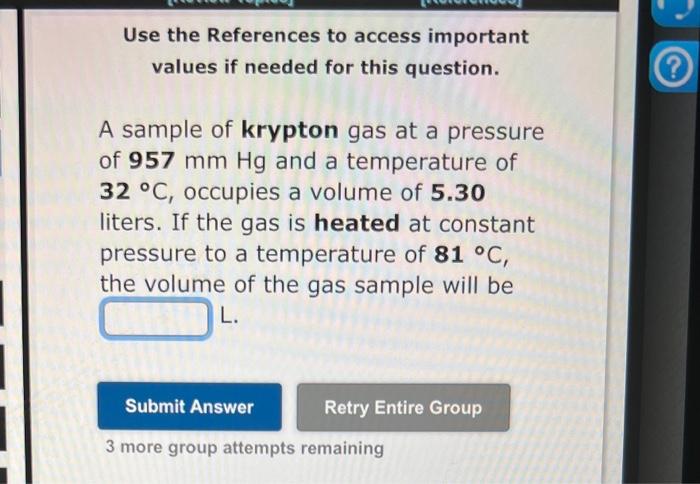






thats the only info given
A sample of krypton gas at a pressure of 957mmHg and a temperature of 32C, occupies a volume of 5.30 liters. If the gas is heated at constant pressure to a temperature of 81C, the volume of the gas sample will be L. 3 more group attempts remaining Use the References to access important values if needed for this question. A sample of xenon gas at a pressure of 0.796atm and a temperature of 226C, occupies a volume of 535mL. If the gas is cooled at constant pressure until its volume is 409mL, the temperature of the gas sample will be C. Use the References to access important values if needed for this question. A sample of hydrogen gas at a pressure of 858mmHg and a temperature of 72C, occupies a volume of 4923.5mL. If the gas is cooled at constant pressure to a temperature of 49C, the volume of the gas sample, in liters, will be L. Use the References to access important values if needed for this question. A helium-filled weather balloon has a volume of 584L at 22.9C and 758mmHg. It is released and rises to an altitude of 7.49km, where the pressure is 348mmHg The volume of the balloon at this altitude is L. Use the References to access important values if needed for this question. A sample of xenon gas occupies a volume of 9.71L at 57.0C and 0.640atm. If it is desired to increase the volume of the gas sample to 10.8L, while increasing its pressure to 0.788 atm, the temperature of the gas sample at the new volume and pressure must be C. Use the References to access important values if needed for this question. 63.0C and 365 torr. If the volume of the gas sample is decreased to 6.47L, while its temperature is increased to 115.0C, the resulting gas pressure will be torr Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


