Answered step by step
Verified Expert Solution
Question
1 Approved Answer
There are various gas laws in addition to the well - known ideal gas law and the van der Waals gas law that we learned
There are various gas laws in addition to the wellknown ideal gas law and the van der Waals gas law that we learned from CHEM Here is another example by Clausius:
where are constants specific to the gas, and the other symbols carry the same meanings as those in the ideal gas law.
a Compute Assume any variables other than and are constants. Such a derivative is more accurately called a partial derivative and is labeled as or
b Compute Assume any variables other than and are constant.
c Compute Assume any variables other than and are constant.
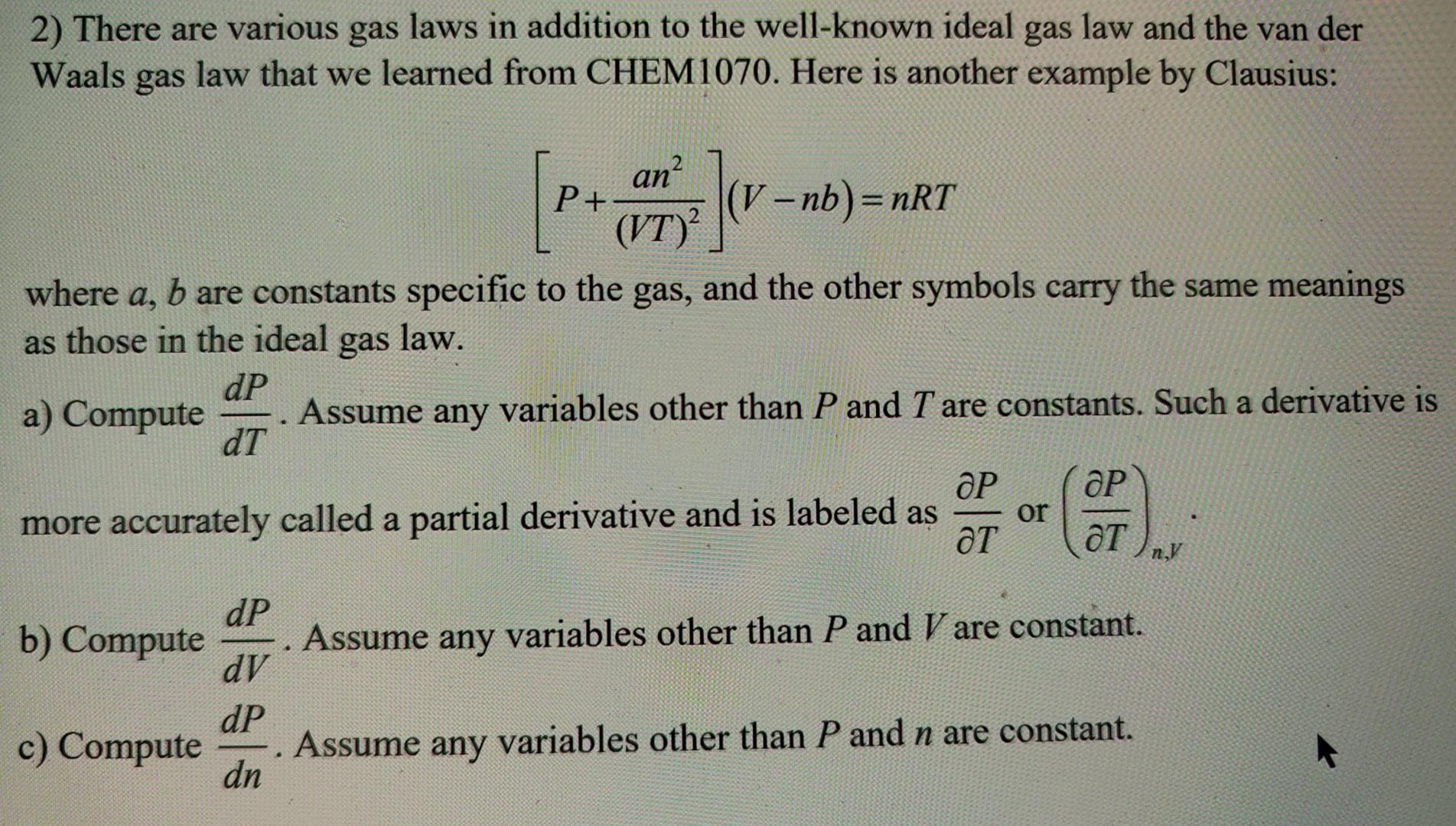
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


