Answered step by step
Verified Expert Solution
Question
1 Approved Answer
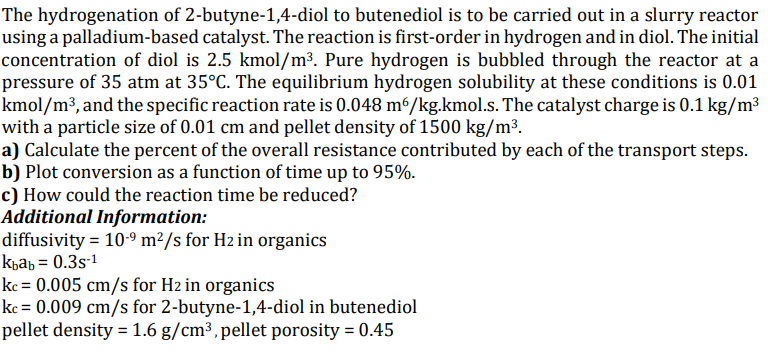
there is no exact and detailed answer in chegg - - - The hydrogenation of 2 - butyne - 1 , 4 - diol to
there is no exact and detailed answer in chegg The hydrogenation of butynediol to butenediol is to be carried out in a slurry reactor
using a palladiumbased catalyst. The reaction is firstorder in hydrogen and in diol. The initial
concentration of diol is kmo Pure hydrogen is bubbled through the reactor at a
pressure of atm at The equilibrium hydrogen solubility at these conditions is
kmo and the specific reaction rate is kmol.s The catalyst charge is
with a particle size of and pellet density of
a Calculate the percent of the overall resistance contributed by each of the transport steps.
b Plot conversion as a function of time up to
c How could the reaction time be reduced?
Additional Information:
diffusivity for in organics
for in organics
for butynediol in butenediol
pellet density pellet porosity
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


