Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Thermal stability varies greatly for the series AlF3 (Melting point = 1291 oC), AlCl3 (Tsub = 180 oC), AlBr3 (Melting point = 97.5 oC) and
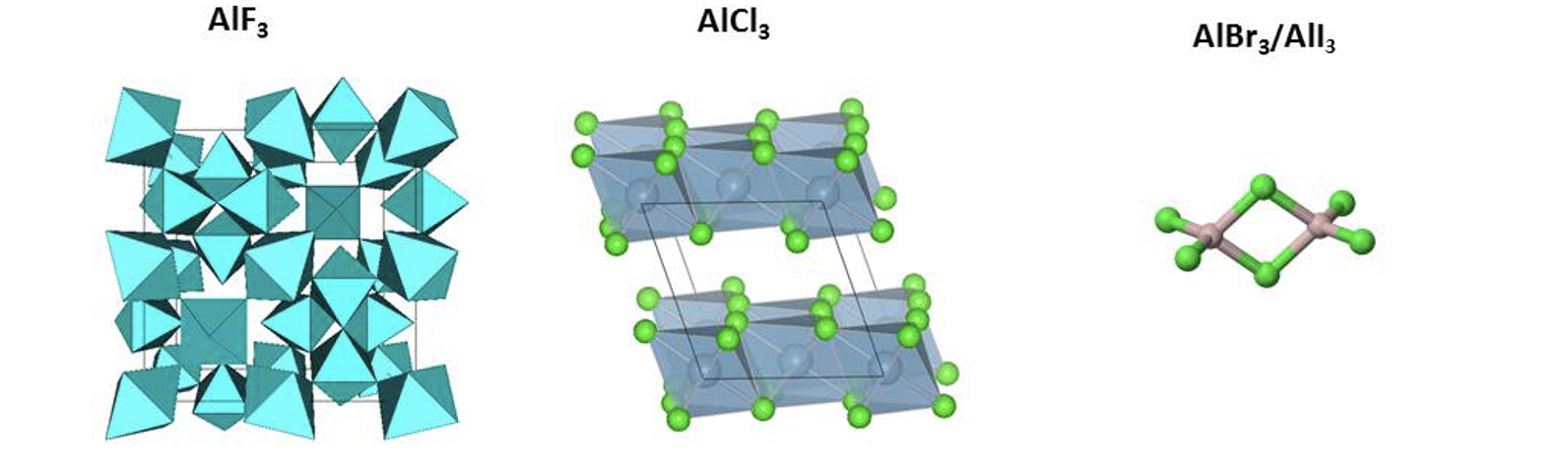
Thermal stability varies greatly for the series AlF3 (Melting point = 1291 oC), AlCl3 (Tsub = 180 oC), AlBr3 (Melting point = 97.5 oC) and AlI3 (Melting point = 189.4 oC). How can this be explained on the basis of crystal structure / molecular structure and chemical bonding conditions (see structure sketches below)?AlF3 AICI AlBrz/Allz
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


