Answered step by step
Verified Expert Solution
Question
1 Approved Answer
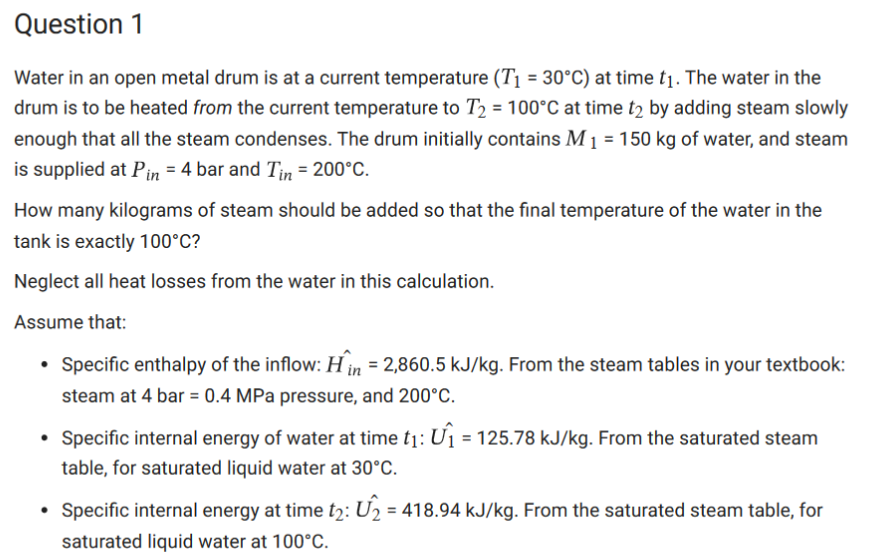
Thermodynamics question: Please explain your answer. Thank you Water in an open metal drum is at a current temperature (T1=30C) at time t1. The water
Thermodynamics question:
Please explain your answer. Thank you
Water in an open metal drum is at a current temperature (T1=30C) at time t1. The water in the drum is to be heated from the current temperature to T2=100C at time t2 by adding steam slowly enough that all the steam condenses. The drum initially contains M1=150kg of water, and steam is supplied at Pin=4 bar and Tin=200C. How many kilograms of steam should be added so that the final temperature of the water in the tank is exactly 100C ? Neglect all heat losses from the water in this calculation. Assume that: - Specific enthalpy of the inflow: Hin^=2,860.5kJ/kg. From the steam tables in your textbook: steam at 4bar=0.4MPa pressure, and 200C. - Specific internal energy of water at time t1:U1^=125.78kJ/kg. From the saturated steam table, for saturated liquid water at 30C. - Specific internal energy at time t2:U2^=418.94kJ/kg. From the saturated steam table, for saturated liquid water at 100CStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


