Answered step by step
Verified Expert Solution
Question
1 Approved Answer
these answers also werent correct. do i get solution for this? A binary mixture of benzene and toluene is to be separated in a continuous
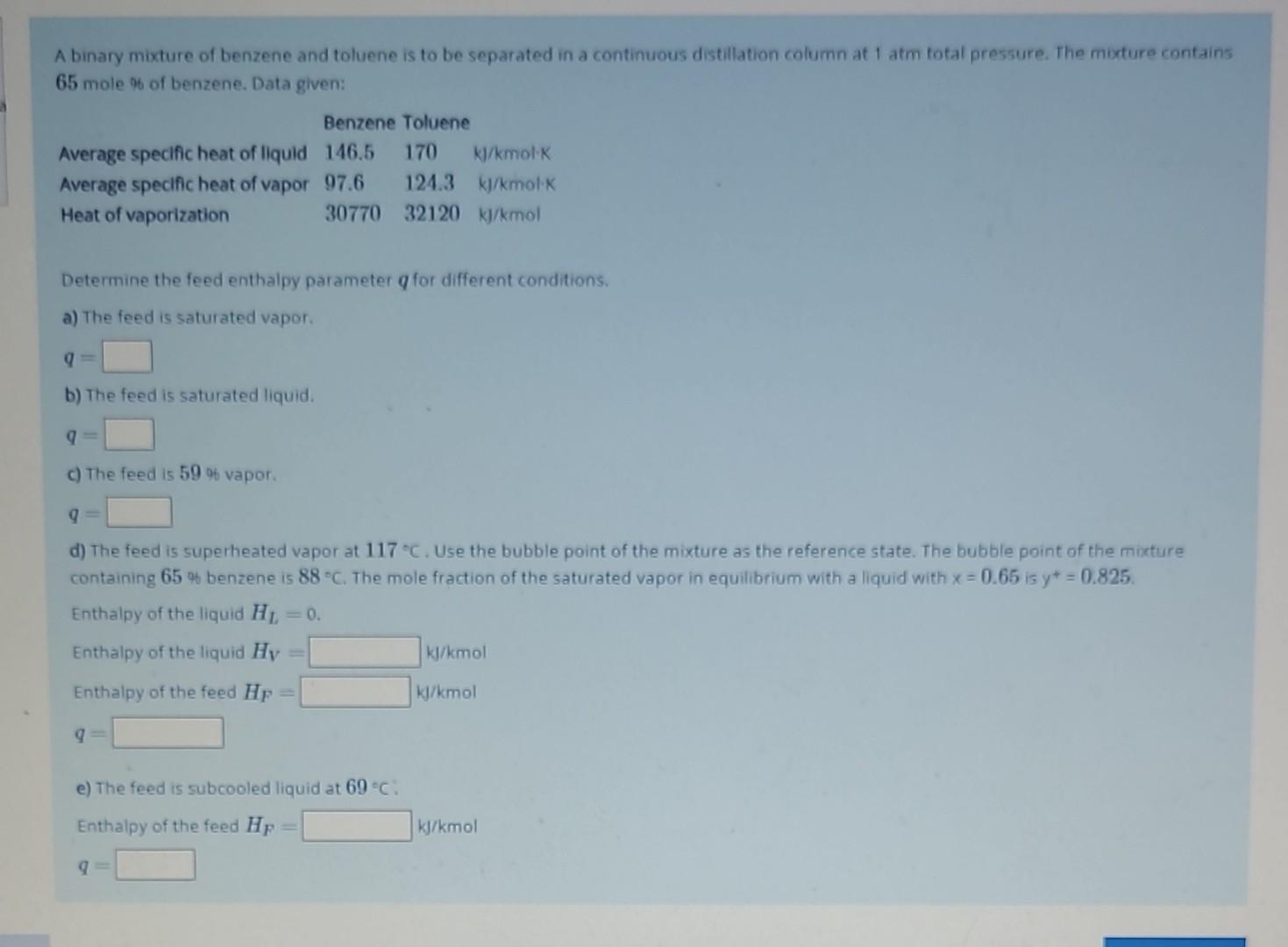
these answers also werent correct. do i get solution for this?
A binary mixture of benzene and toluene is to be separated in a continuous distillation column at 1 atm fotal pressure. The mixture contains 65 mole % of benzene. Data given: Determine the feed enthalpy parameter q for different conditions. a) The feed is saturated vapor. q= b) The feed is saturated liquid. q= c) The feed is 59 of vapor. q= d) The feed is superheated vapor at 117C. Use the bubble point of the mixture as the reference state. The bubble point of the mixture containing 65 benzene is 88C. The mole fraction of the saturated vapor in equilibrium with a liquid with x=0.65 is y=0.825. Enthalpy of the liquid HL=0. Enthalpy of the liquid HV= kJ/kmol Enthalpy of the feed HF= k/kmol q= e) The feed is subcooled liquid at 69C : Enthalpy of the feed HF= kk/kmol q=Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


