Answered step by step
Verified Expert Solution
Question
1 Approved Answer
these are more clear and include numbers, thank you in advance! 1. An unknown hydrocarbon with the molecular formula C2H2 is treated with one equivalent
these are more clear and include numbers, thank you in advance! 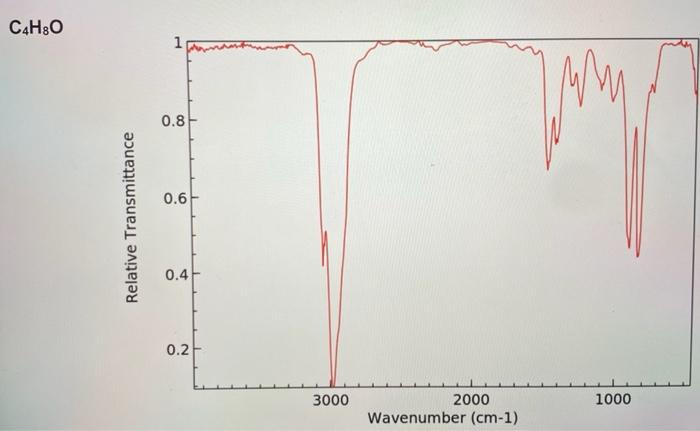
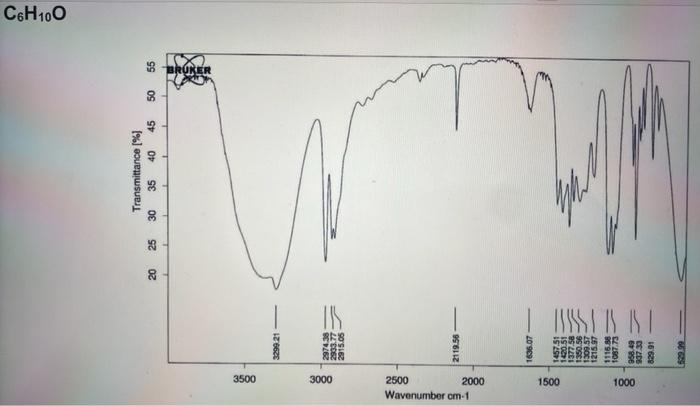
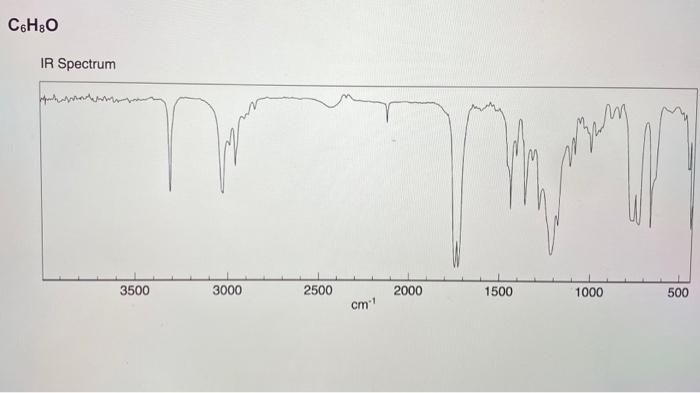
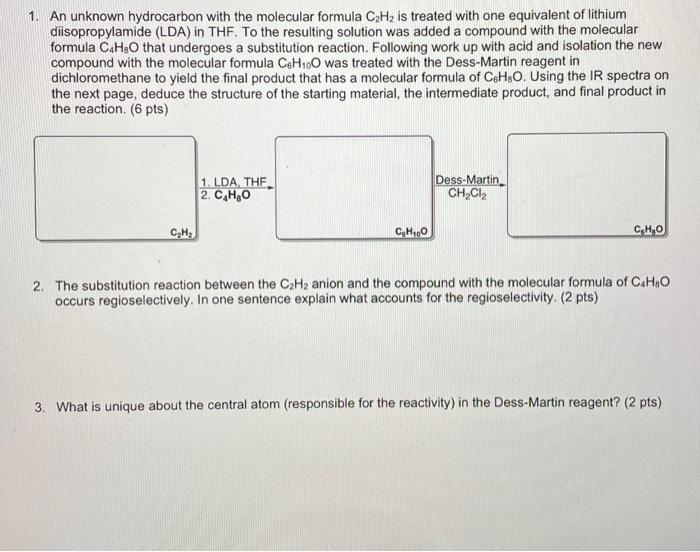
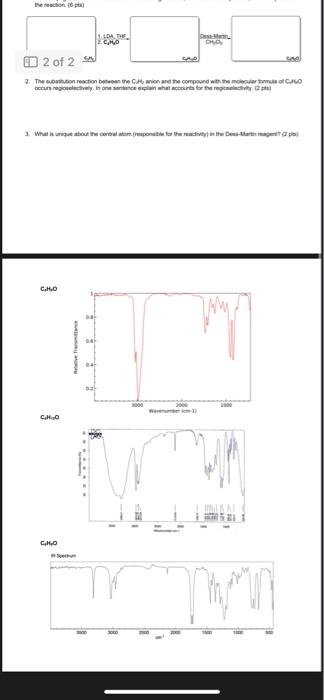
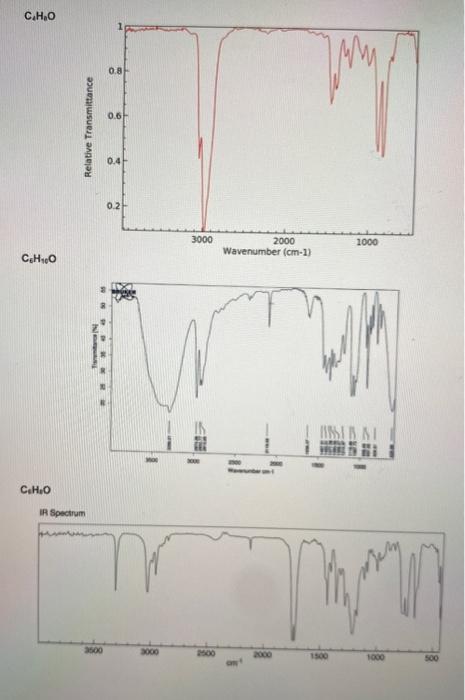
1. An unknown hydrocarbon with the molecular formula C2H2 is treated with one equivalent of lithium diisopropylamide (LDA) in THF. To the resulting solution was added a compound with the molecular formula C4H8O that undergoes a substitution reaction. Following work up with acid and isolation the new compound with the molecular formula C6H10O was treated with the Dess-Martin reagent in dichloromethane to yield the final product that has a molecular formula of C6H8O. Using the IR spectra on the next page, deduce the structure of the starting material, the intermediate product, and final product in the reaction. (6 pts) 2. The substitution reaction between the C2H2 anion and the compound with the molecular formula of C4H8O occurs regioselectively. In one sentence explain what accounts for the regioselectivity. (2 pts) 3. What is unique about the central atom (responsible for the reactivity) in the Dess-Martin reagent? (2 pts) C4H8O C6H10O 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


