Answered step by step
Verified Expert Solution
Question
1 Approved Answer
These three photos above are information given with the experiment.It may contain information that can help in answering the questions below. This is one question

These three photos above are information given with the experiment.It may contain information that can help in answering the questions below.

This is one question consisting of Part a and part b. Please respond to both questions. I appreciate the help you may offer.Thank you in advance.
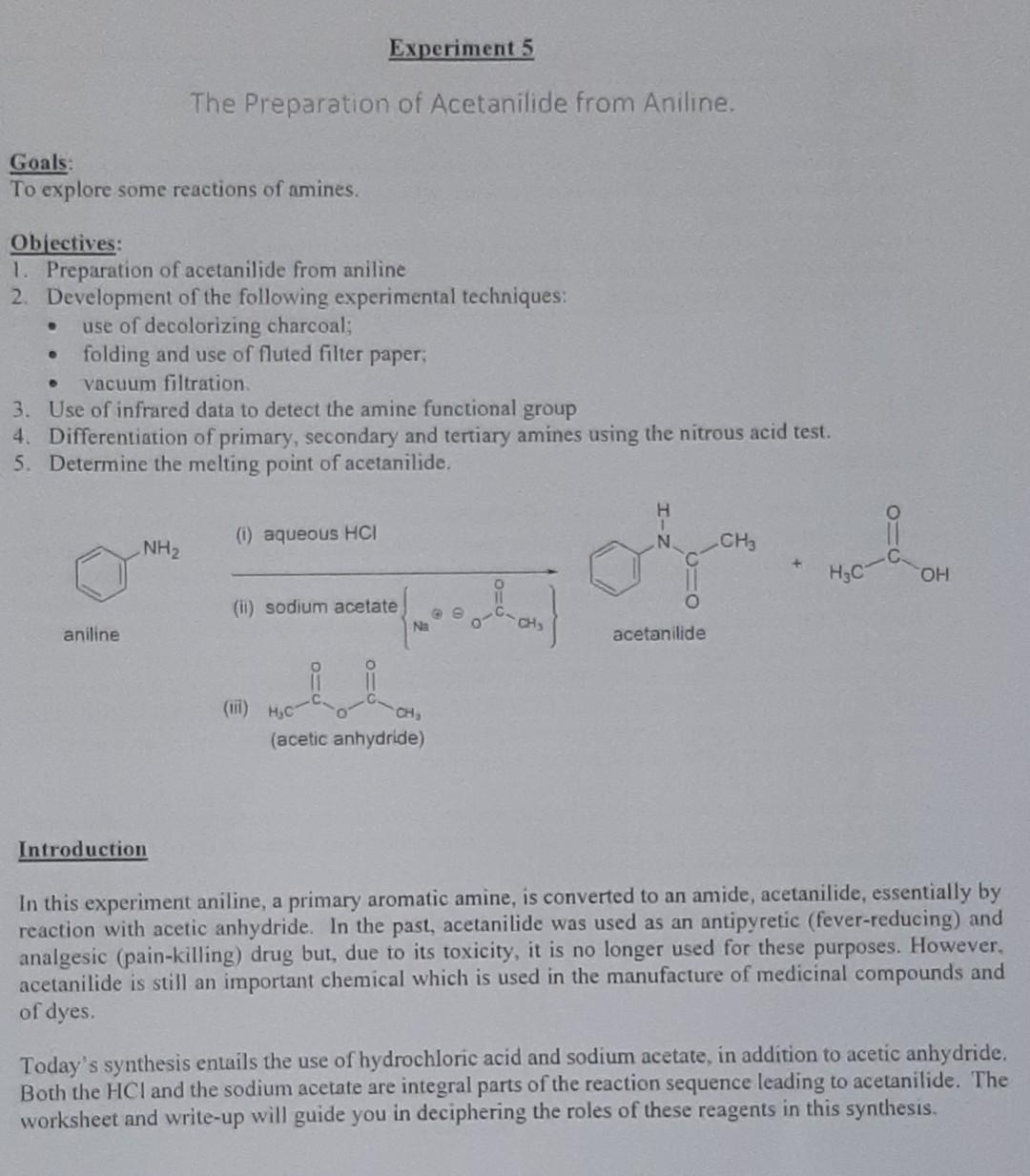
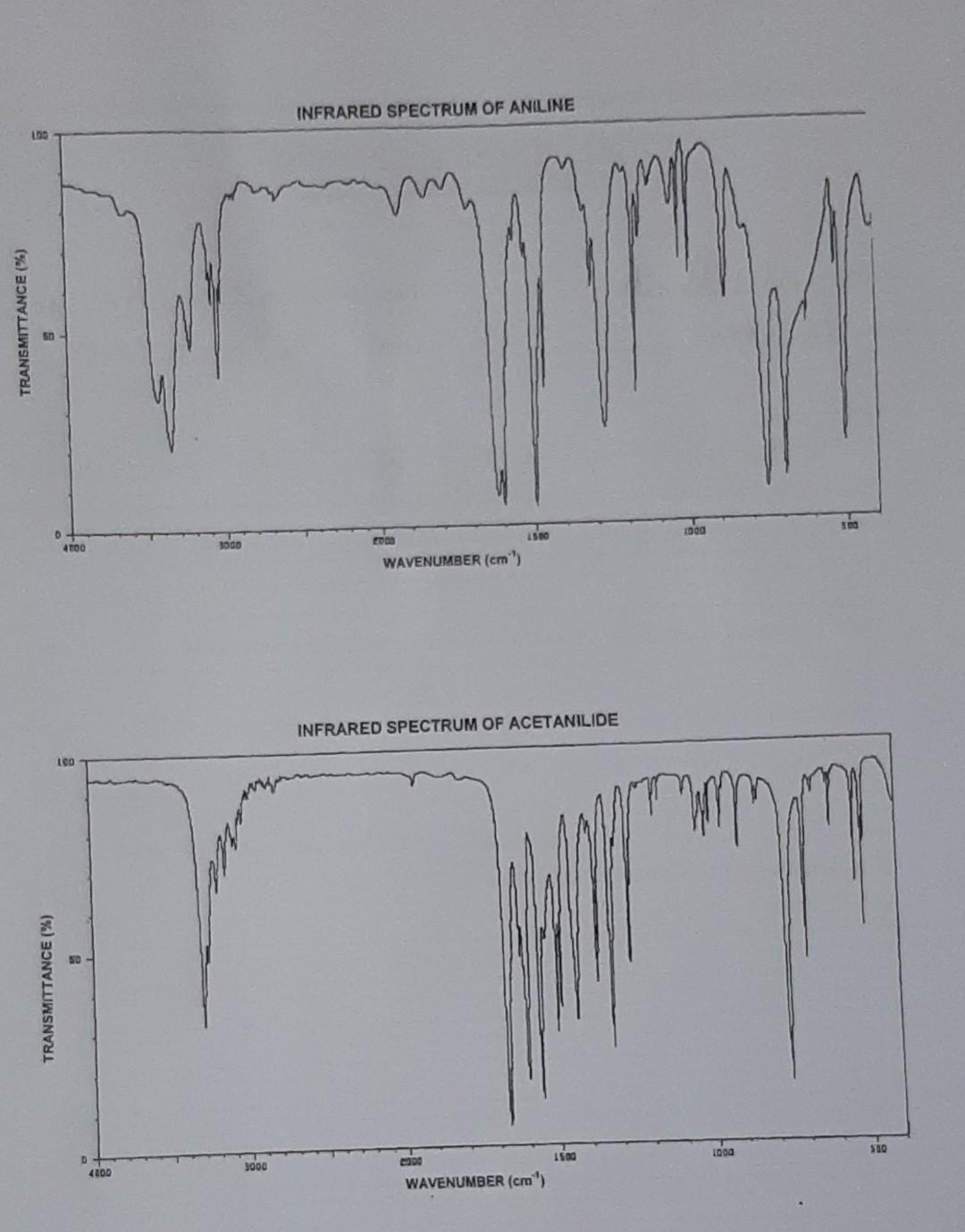
Experiment 5 The Preparation of Acetanilide from Aniline. Goals: To explore some reactions of amines. . Objectives: 1. Preparation of acetanilide from aniline 2. Development of the following experimental techniques: use of decolorizing charcoal; folding and use of fluted filter paper, vacuum filtration 3. Use of infrared data to detect the amine functional group 4. Differentiation of primary, secondary and tertiary amines using the nitrous acid test. 5. Determine the melting point of acetanilide. H (1) aqueous HCI N NH2 CH3 H3C (11) sodium acetate other Na aniline acetanilide (ii) CH (acetic anhydride) Introduction In this experiment aniline, a primary aromatic amine, is converted to an amide, acetanilide, essentially by reaction with acetic anhydride. In the past, acetanilide was used as an antipyretic (fever-reducing) and analgesic (pain-killing) drug but, due to its toxicity, it is no longer used for these purposes. However, acetanilide is still an important chemical which is used in the manufacture of medicinal compounds and of dyes. Today's synthesis entails the use of hydrochloric acid and sodium acetate, in addition to acetic anhydride. Both the HCI and the sodium acetate are integral parts of the reaction sequence leading to acetanilide. The worksheet and write-up will guide you in deciphering the roles of these reagents in this synthesis. A Safety Notes You must wear eye protection at all times. In the event that any reagent used in this investigation comes in contact with your skin or eyes, wash the affected area immediately with lots of water. Notify your instructor. Aniline is toxic and can be absorbed through the skin. Use the fume hood. Concentrated hydrochloric acid can cause severe burns. Acetic anhydride is lachrymaton Procedure To 30 cm water in a 100 cm Erlenmeyer flask add 1 cmconcentrated hydrochloric acid with mixing. In the fume hood add 1 cm'aniline (density 1.02 g cm-3) and swirl the mixture. If the solution is coloured, add a small amount of decolorizing charcoal, swirl the flask for about one minute, and filter off the carbon using a Nuted filter paper (Appendix 7). In a separate container dissolve 1.5 g sodium acetate in 5 cm water. Warm the anilinium chloride solution to 50C on a water bath and add 2 cm of acetic anhydride (density 1.08 g cm). Swirl to effect dissolution and add the aqueous sodium acctate quickly. Swirl the flask a couple of times and set it in an ice- bath for 20 min. Filter, with suction, the crystals of the amide formed and wash with a small amount of ice-cold water. Dry the material between filter papers and submit your sample for assessment. Determine the melting point of the product. Inspect the infrared spectra of the starting material and product. In your write-up you will be asked to list the major bands and the groups which give rise to them (see Appendix 3) and to relate these data to the reaction which has occurred. INFRARED SPECTRUM OF ANILINE TRANSMITTANCE (%) QO D 4100 1000 toa 15 WAVENUMBER (cm) INFRARED SPECTRUM OF ACETANILIDE LCO TRANSMITTANCE (%) 500 1500 1000 3000 4000 WAVENUMBER (cm) Write a mechanism for the reaction between aniline and acetic anhydride. a (5) Brief discussion (This should include, but not be limited to, an outline of the merits and or the drawbacks of the methods and techniques used in this synthesis and the factors which could have affected the yield and quality of your product.)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


