Question
Think about the conditions where CH3OH gas (1mol) is occupied in the cubic box: * Dimensions: 3m x 3m x 3m * Temperature inside the
Think about the conditions where CH3OH gas (1mol) is occupied in the cubic box:
* Dimensions: 3m x 3m x 3m
* Temperature inside the box is 20K
* R = 0.0812 L atm/mol K
* CH3OH molecules behave like the ideal gas conditions.
If CH3OH is an IDEAL GAS, calculate a pressure in this chamber numerically by converting a unit of volume as 1m^3 = 10^3 L so that pressure is in the unit of ATM.
AND, if this gas CH3OH, in this case the molecule hits the wall with the velocity of Vx, it bounces back to the opposite direction non-elastically and the velocity decrease to 0.5 vx.
Derive a pressure of single molecule of CH3OH by using the micro-particle model. The answer must contain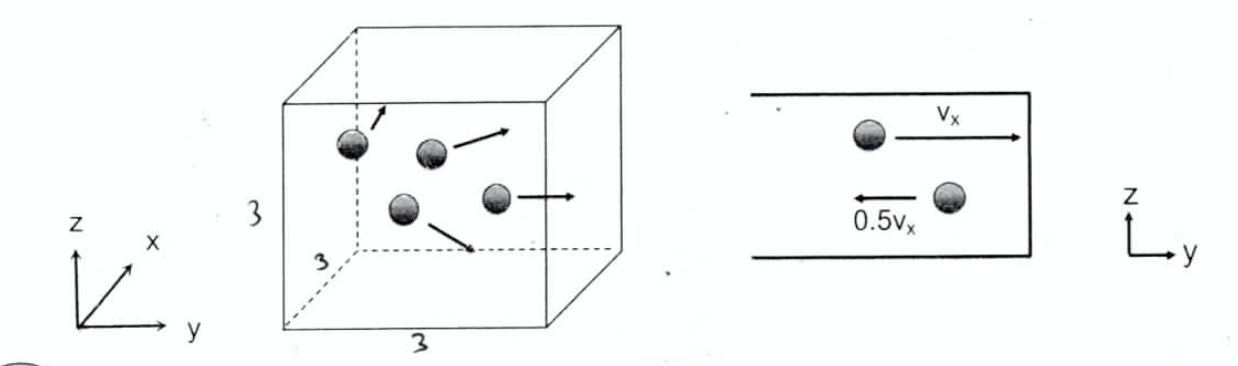 m, V and Vx.
m, V and Vx.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


