Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This graph shows how the vapor pressure of three liquids varies with temperature: A 900 800 700 600 vapor pressure, torr 500 400 300 200
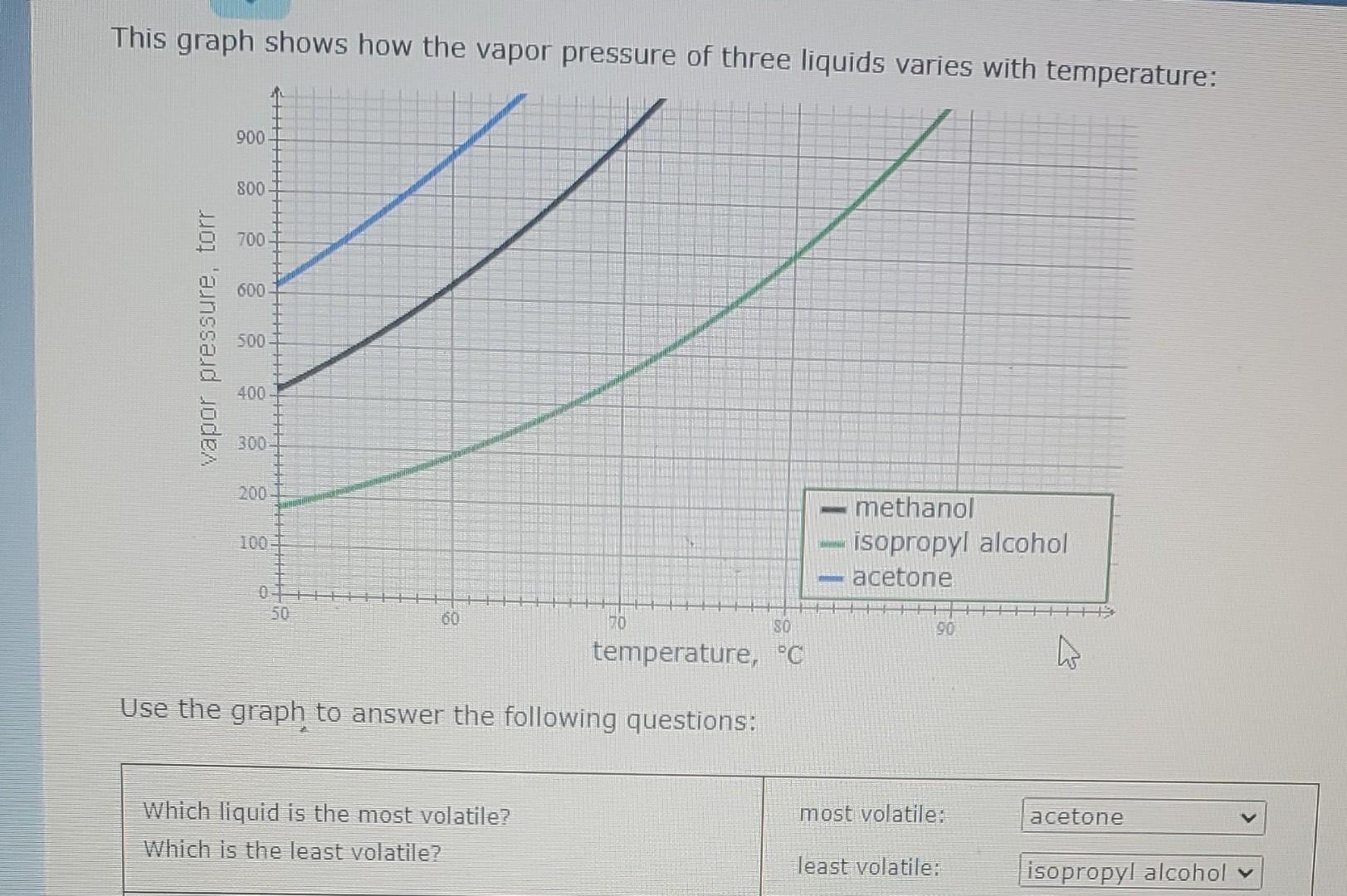

This graph shows how the vapor pressure of three liquids varies with temperature: A 900 800 700 600 vapor pressure, torr 500 400 300 200 100 methanol isopropyl alcohol acetone 50 60 70 180 90 temperature, C h Use the graph to answer the following questions: most volatile: acetone Which liquid is the most volatile? Which is the least volatile? least volatile: isopropyl alcohol Use the graph to answer the following questions: Which liquid is the most volatile? Which is the least volatile? most volatile: acetone least volatile: isopropyl alcohol methanol: 56 C What is the normal boiling point of each liquid? Note: your answer must be within 1C of the exact answer to be graded correct. isopropyl alcohol: 65 C acetone: 78 C O more Suppose a beaker of isopropyl alcohol is put inside a sealed tank containing isopropyl alcohol gas at 66. degree C and 627. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? less the same
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


