Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This is a subjective question, hence you have to write your answer in the Text-Field given below. In the production of SO3,100kmol of SO2 and
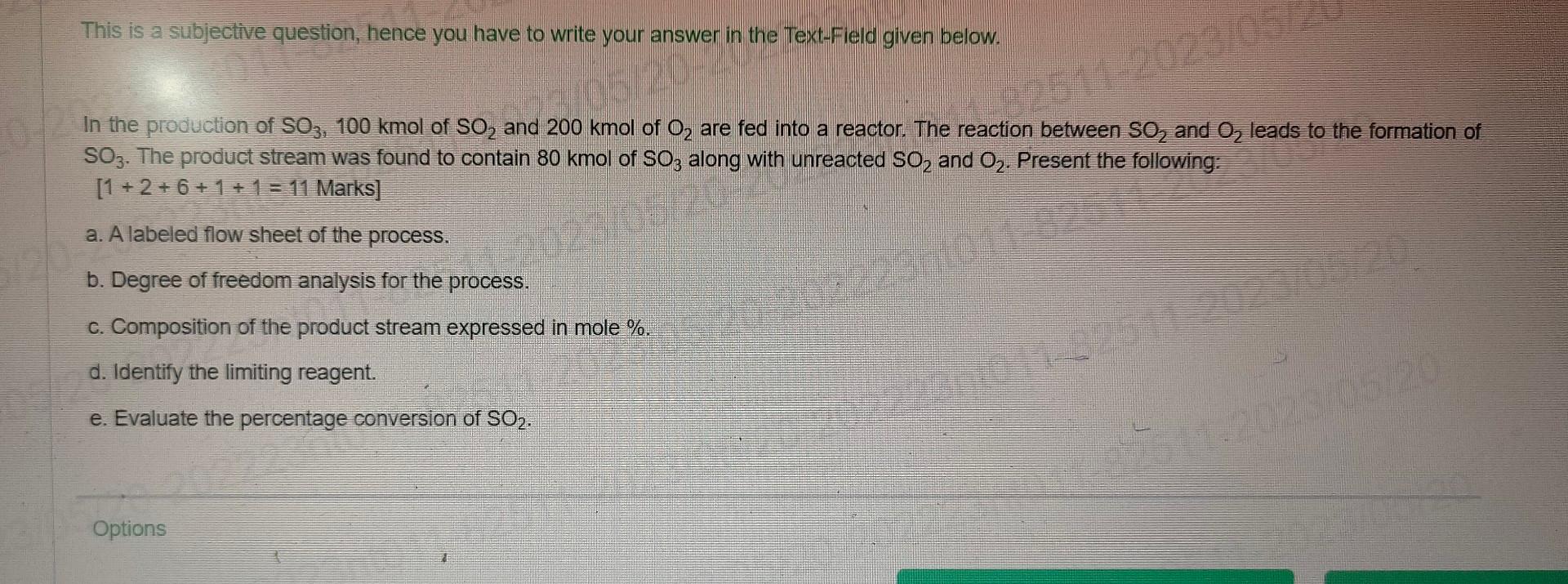
This is a subjective question, hence you have to write your answer in the Text-Field given below. In the production of SO3,100kmol of SO2 and 200kmol of O2 are fed into a reactor. The reaction between SOO2 and O2 leads to the formation of SO3. The product stream was found to contain 80kmol of SO3 along with unreacted SO2 and O2. Present the following: [1+2+6+1+1=11Marks] a. A labeled flow sheet of the process. b. Degree of freedom analysis for the process. c. Composition of the product stream expressed in mole %. d. Identify the limiting reagent. e. Evaluate the percentage conversion of SO2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


