Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This is Hess's Law Please help me on 7,8,9,10,11 and also help me fill out in the table ( Experimental value, Accepted value, Percent error)
This is Hess's Law 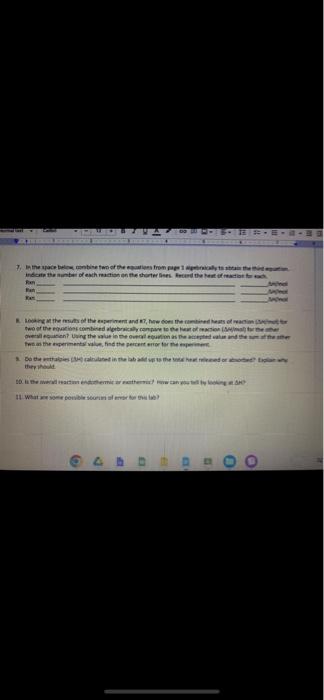
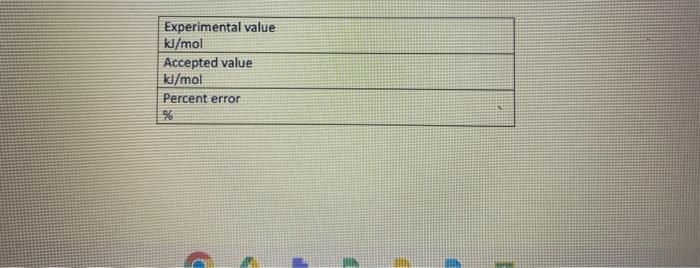
Please help me on 7,8,9,10,11 and also help me fill out in the table ( Experimental value, Accepted value, Percent error)
Note in question 7: Combine two of equations algebraically to obtain the third reactions
I include everything you guys need
Please help me




Here is a question 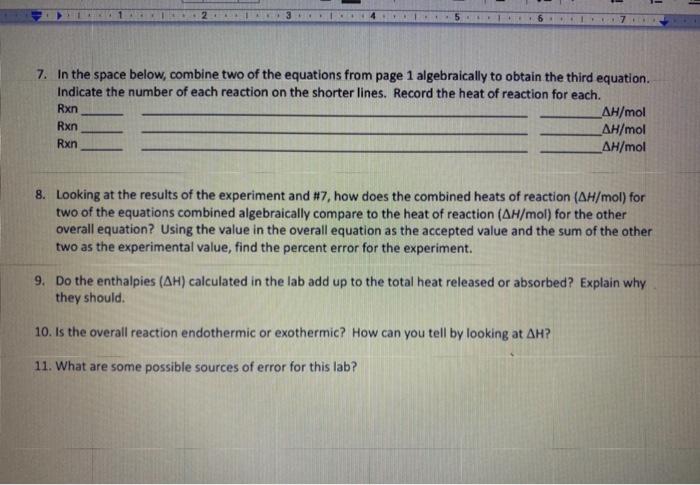

They are all information you need
Note:
In question 7: They asking two equations from reaction 1 and 2 to obtain the reaction 3
What do you need more?
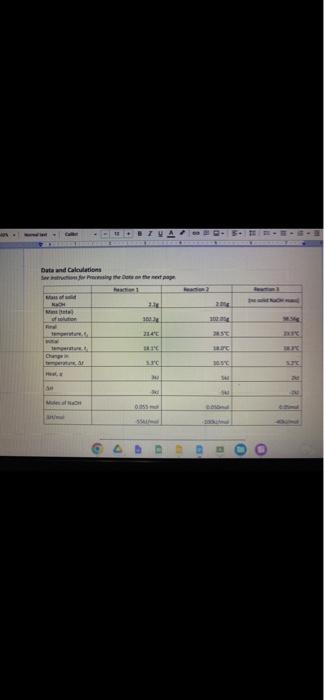
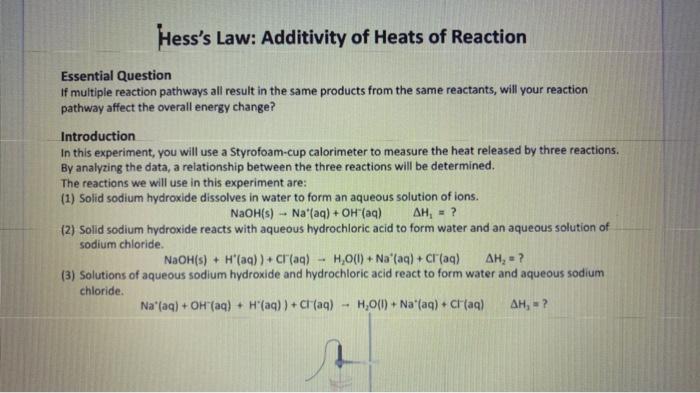
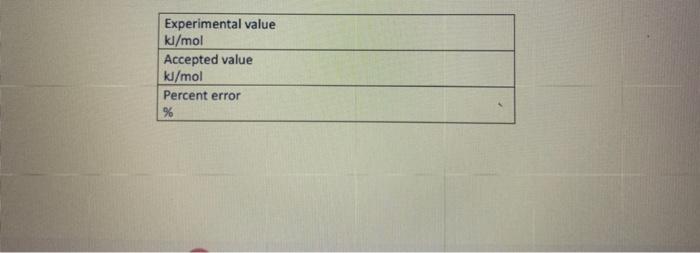
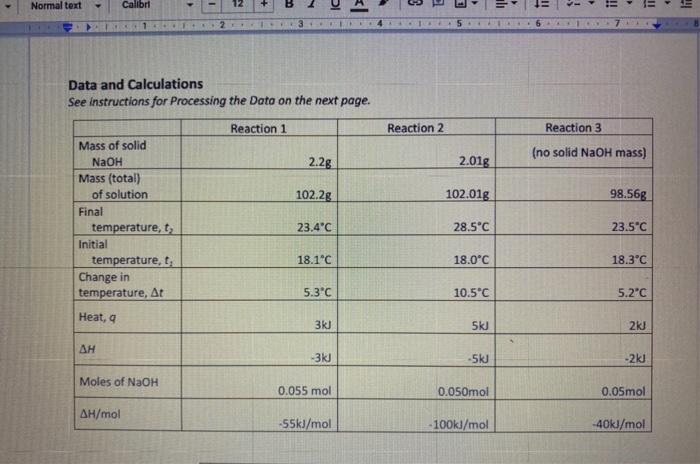
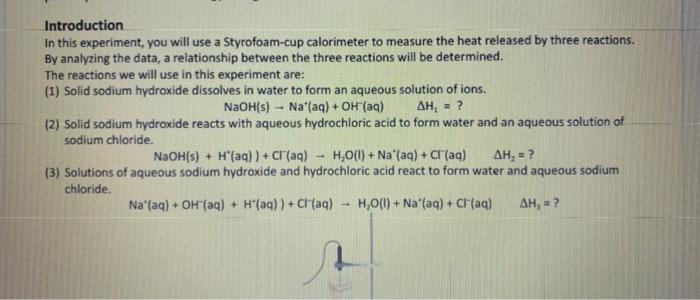
Fin Fint Fin Burp pavid. Experimental value kJ/mol Accepted value kj/mol Percent error % Data and Calodotions Hess's Law: Additivity of Heats of Reaction Essential Question If multiple reaction pathways all result in the same products from the same reactants, will your reaction pathway affect the overall energy change? Introduction In this experiment, you will use a Styrofoam-cup calorimeter to measure the heat released by three reactions. By analyzing the data, a relationship between the three reactions will be determined. The reactions we will use in this experiment are: (1) Solid sodium hydroxide dissolves in water to form an aqueous solution of ions. NaOH(s)Na(aq)+OH(aq)H1=? (2) Solid sodium hydroxide reacts with aqueous hydrochloric acid to form water and an aqueous solution of sodium chloride. NaOH(s)+H(aq))+Cr(aq)H2O(l)+Na(aq)+Cr(aq)H2=? (3) Solutions of aqueous sodium hydroxide and hydrochloric acid react to form water and aqueous sodium chloride. Na(aq)+OH(aq)+H(aq))+Cl(aq)H2O(l)+Na(aq)+Cl(aq)H2=? 7. In the space below, combine two of the equations from page 1 algebraically to obtain the third equation. Indicate the number of each reaction on the shorter lines. Record the heat of reaction for each. Rxn Rxn Rxn 8. Looking at the results of the experiment and #7, how does the combined heats of reaction (H/mol) for two of the equations combined algebraically compare to the heat of reaction (H/mol) for the other overall equation? Using the value in the overall equation as the accepted value and the sum of the other two as the experimental value, find the percent error for the experiment. 9. Do the enthalpies (H) calculated in the lab add up to the total heat released or absorbed? Explain why they should. 10. Is the overall reaction endothermic or exothermic? How can you tell by looking at H ? 11. What are some possible sources of error for this lab? \begin{tabular}{|l|} \hline Experimental value \\ kJ/mol \\ \hline Accepted value \\ kJ/mol \\ \hline Percent error % \\ \hline \end{tabular} Data and Calculations See instructions for Processing the Dato on the next page. Introduction In this experiment, you will use a Styrofoam-cup calorimeter to measure the heat released by three reactions. By analyzing the data, a relationship between the three reactions will be determined. The reactions we will use in this experiment are: (1) Solid sodium hydroxide dissolves in water to form an aqueous solution of ions. NaOH(s)Na+(aq)+OH(aq)H1=? (2) Solid sodium hydroxide reacts with aqueous hydrochloric acid to form water and an aqueous solution of sodium chloride. NaOH(s)+H(aq))+Cr(aq)H2O(l)+Na(aq)+Cr(aq)H2=? (3) Solutions of aqueous sodium hydroxide and hydrochloric acid react to form water and aqueous sodium chloride. Na+(aq)+OH(aq)+H(aq))+Cl(aq)H2O(l)+Na(aq)+Cl(aq)H1= Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


