Answered step by step
Verified Expert Solution
Question
1 Approved Answer
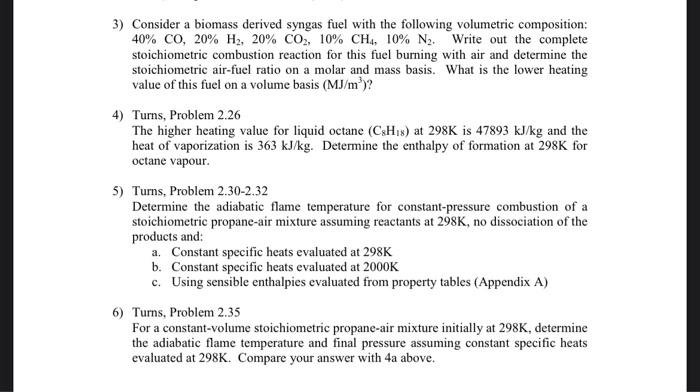
This is on fundamentals of combustion. ( Background in thermodynamics) 3) Consider a biomass derived syngas fuel with the following volumetric composition: 40% Co, 20%
This is on fundamentals of combustion. ( Background in thermodynamics) 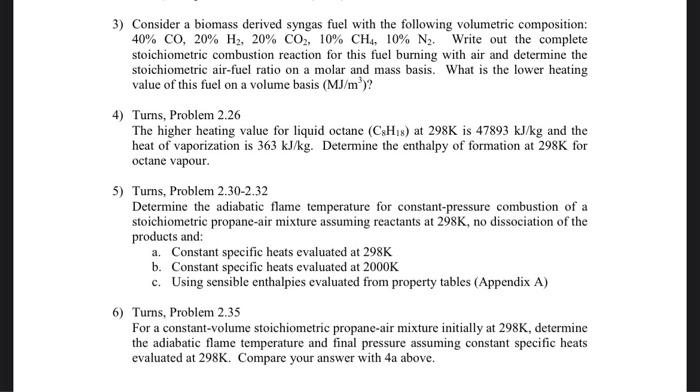
3) Consider a biomass derived syngas fuel with the following volumetric composition: 40% Co, 20% H2, 20% CO2, 10% CH4, 10% N. Write out the complete stoichiometric combustion reaction for this fuel burning with air and determine the stoichiometric air-fuel ratio on a molar and mass basis. What is the lower heating value of this fuel on a volume basis (MJ/m)? 4) Turns, Problem 2.26 The higher heating value for liquid octane (CH) at 298K is 47893 kJ/kg and the heat of vaporization is 363 kJ/kg. Determine the enthalpy of formation at 298K for octane vapour. 5) Turns, Problem 2.30-2.32 Determine the adiabatic flame temperature for constant-pressure combustion of a stoichiometric propane-air mixture assuming reactants at 298K, no dissociation of the products and a. Constant specific heats evaluated at 298K b. Constant specific heats evaluated at 2000K c. Using sensible enthalpies evaluated from property tables (Appendix A) 6) Turns, Problem 2.35 For a constant-volume stoichiometric propane-air mixture initially at 298K, determine the adiabatic flame temperature and final pressure assuming constant specific heats evaluated at 298K. Compare your answer with 4a above. 3) Consider a biomass derived syngas fuel with the following volumetric composition: 40% CO, 20% H2, 20% CO2, 10% CH4, 10% N2. Write out the complete stoichiometric combustion reaction for this fuel burning with air and determine the stoichiometric air-fuel ratio on a molar and mass basis. What is the lower heating value of this fuel on a volume basis (MJ/m)? 4) Turns, Problem 2.26 The higher heating value for liquid octane (CxHys) at 298K is 47893 kJ/kg and the heat of vaporization is 363 kJ/kg. Determine the enthalpy of formation at 298K for octane vapour. 5) Turns, Problem 2.30-2.32 Determine the adiabatic flame temperature for constant-pressure combustion of a stoichiometric propane-air mixture assuming reactants at 298K, no dissociation of the products and a. Constant specific heats evaluated at 298K b. Constant specific heats evaluated at 2000K c. Using sensible enthalpies evaluated from property tables (Appendix A) 6) Turns, Problem 2.35 For a constant-volume stoichiometric propane-air mixture initially at 298K, determine the adiabatic flame temperature and final pressure assuming constant specific heats evaluated at 298K. Compare your answer with 4a above 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


