Answered step by step
Verified Expert Solution
Question
1 Approved Answer
this is one question with two parts part 1 part two: Determine the standard enthalpy change for each of the following reactions. Report your answers
this is one question with two parts 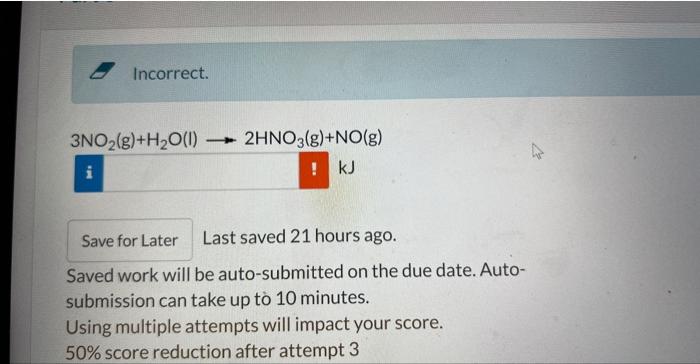
Ethanol, CH3CH2OH, is used as a gasoline additive because it boosts octane ratings. Gasoline that contains ethanol is known as gasohol. Calculate the amount of energy released by burning one gallon of ethanol. The density of ethanol is 0.787g/mL 1 gallon =3.785L AH(CO2(g))=393.5kJ/mol AH2(H2O(D)=285.83kJ/mol TARIF AVERAGE BOND ENERGIES * Al values are in kilojoules per mole (kJ/mol) and are rounded to ue meares s nmm. C=O bond energy in CO2 3NO2(g)+H2O(l)2HNO3(g)+NO(g) kJ Last saved 21 hours ago. Saved work will be auto-submitted on the due date. Autosubmission can take up t 10 minutes. Using multiple attempts will impact your score. 50% score reduction after attempt 3 part 1 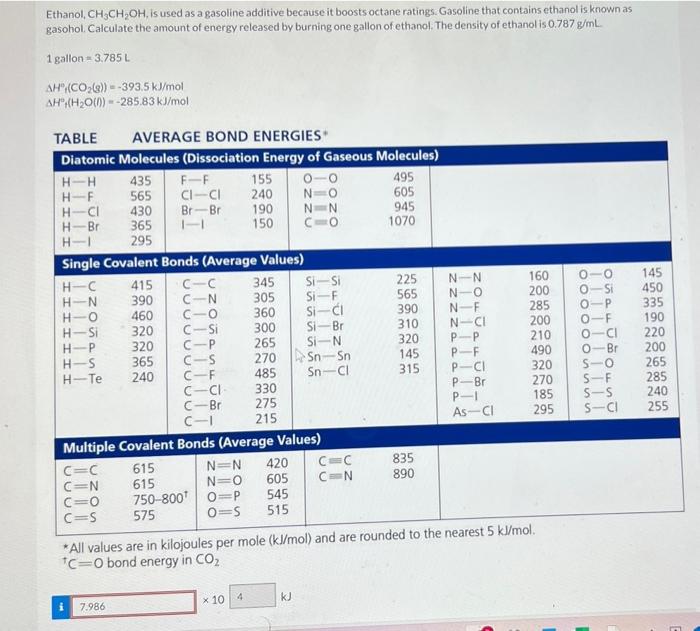

part two:
Determine the standard enthalpy change for each of the following reactions. Report your answers to the nearest 0.1 kJ.

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


