Question
This is only one question but multi-parts connected. If not permitted I can submit another question for the remaining unanswered. Thank you! Question 1 :
This is only one question but multi-parts connected. If not permitted I can submit another question for the remaining unanswered. Thank you!
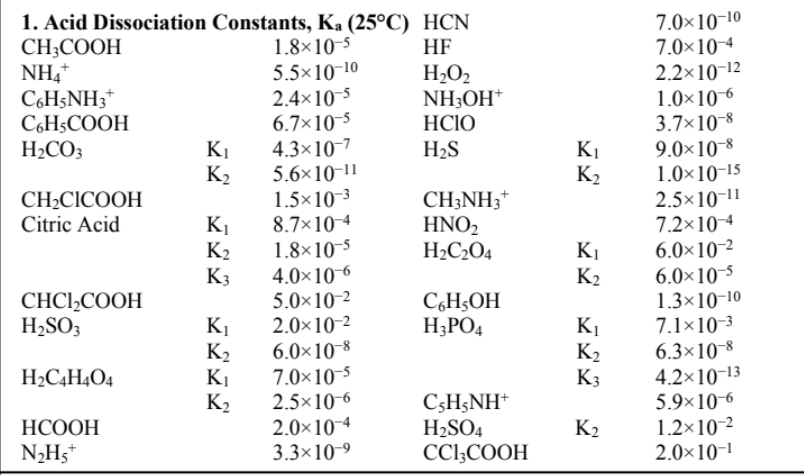
Question 1 : Based on the data sheet given,
A buffer of pH 5.09 is required.
(a) Select from the Data Sheet the most appropriate acid/conjugate base combination.
(b) What is the [acid]:[base] ratio in that buffer?
(c) If, in the final buffer, the concentration of base is 0.50 M, calculate the amounts (in g) of acid and base needed to prepare 1.0 L of buffer. If a salt is needed, pick either the Cl salt or the Li+ salt.
(d) 400 mL of the buffer is taken. If 4.00 mL of 4.0 M NaOH (MM = 40.0 gmol1 is added to the buffer, what is the new pH?
K K2 1. Acid Dissociation Constants, Ka (25C) HCN CH3COOH 1.8x10-5 HF NH4+ 5.5x10-10 H202 C6H5NH3+ 2.4x10-5 NH3OH C6H3COOH 6.7x10-5 HCIO H2CO3 K 4.3x10-7 HS K 5.6x10-11 CHCICOOH 1.5x10-3 CH3NH3- Citric Acid K 8.7x10-4 HNO2 K2 1.8x10-5 H2C204 K3 4.0x10-6 CHCI,COOH 5.0*10-2 C6H5OH H2SO3 K 2.0x10-2 H3PO4 6.0x10-8 H2C4H404 K 7.0x10-5 K2 2.5x10-6 CsH NH HCOOH 2.0x10-4 H2SO4 N2H5+ 3.3x10-9 CCI3COOH 7.0*10-10 7.0*10-4 2.2x10-12 1.0x10-6 3.7x10-8 9.0x10-8 1.0x10-15 2.5x 10-11 7.2x10-4 6.0x10-2 6.0x10-5 1.3x10-10 7.1x10-3 6.3x10-8 4.2x10-13 5.9x10-6 1.2x 10-2 2.0x10-1 K K2 K2 K K2 K; K2Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


