Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This is the diels Alder post lab Mass of anthracene: 0 . 8 3 8 7 g Mass of maleic anhydride: 0 . 4 4
This is the diels Alder post lab
Mass of anthracene: g
Mass of maleic anhydride: g
Final mass: g
Melting point: to degrees C
Please need asap:
Reaction
Draw the overall reaction of anthracene and maleic anhydride. pts
Describe the atom economy for this reaction. You will likely need to look up and cite a source for this. Textbooks or reputable websites are fine
Results pts
Show your calculations for theoretical and percent yield based on your values from lab. Include the stoichiometric conversion. List your melting point with your percent yield.
Analysis
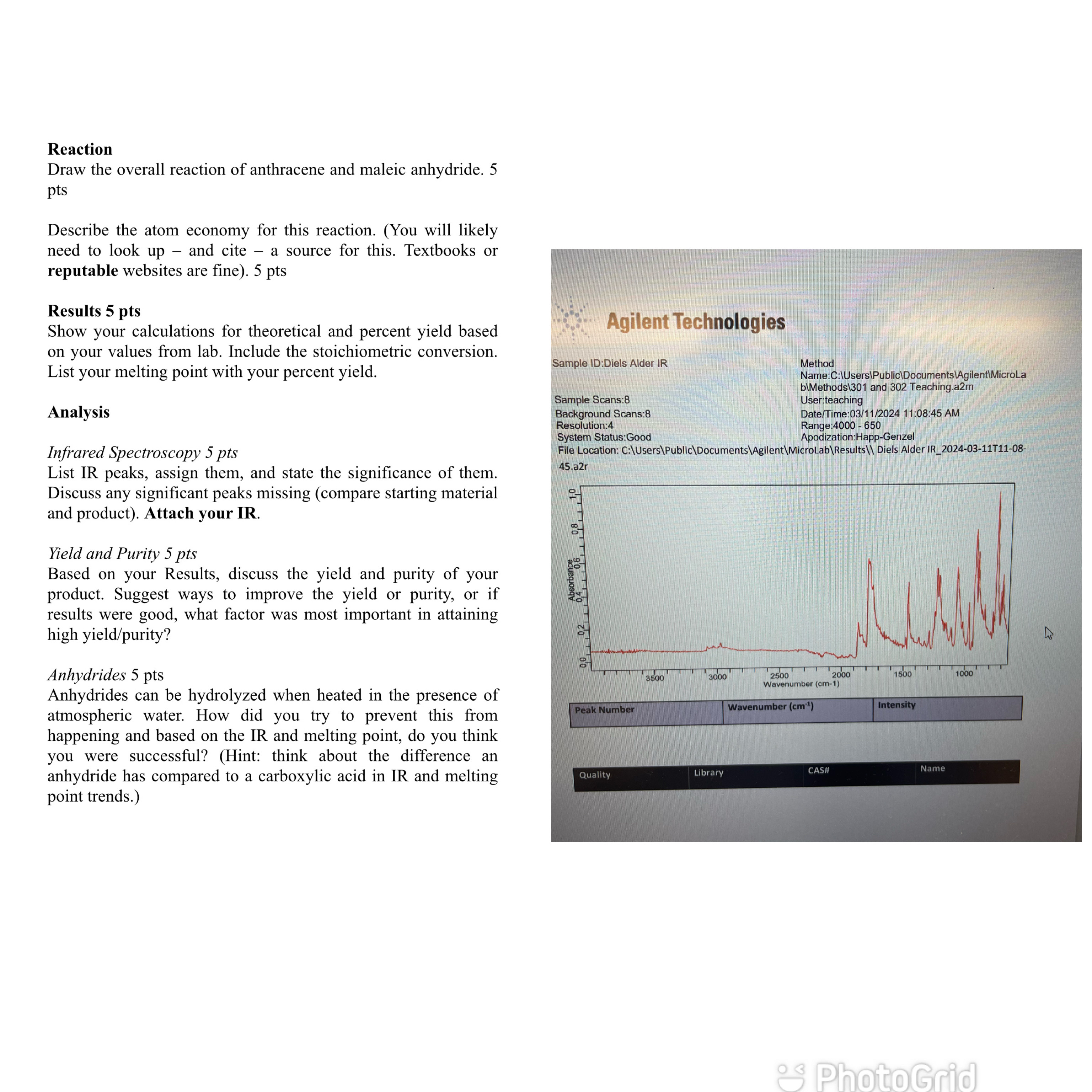
Infrared Spectroscopy pts
List IR peaks, assign them, and state the significance of them. Discuss any significant peaks missing compare starting material and product Attach your IR
Yield and Purity pts
Based on your Results, discuss the yield and purity of your product. Suggest ways to improve the yield or purity, or if results were good, what factor was most important in attaining high yieldpurity
Anhydrides pts
Anhydrides can be hydrolyzed when heated in the presence of atmospheric water. How did you try to prevent this from happening and based on the IR and melting point, do you think you were successful? Hint: think about the difference an anhydride has compared to a carboxylic acid in IR and melting point trends.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


