Answered step by step
Verified Expert Solution
Question
1 Approved Answer
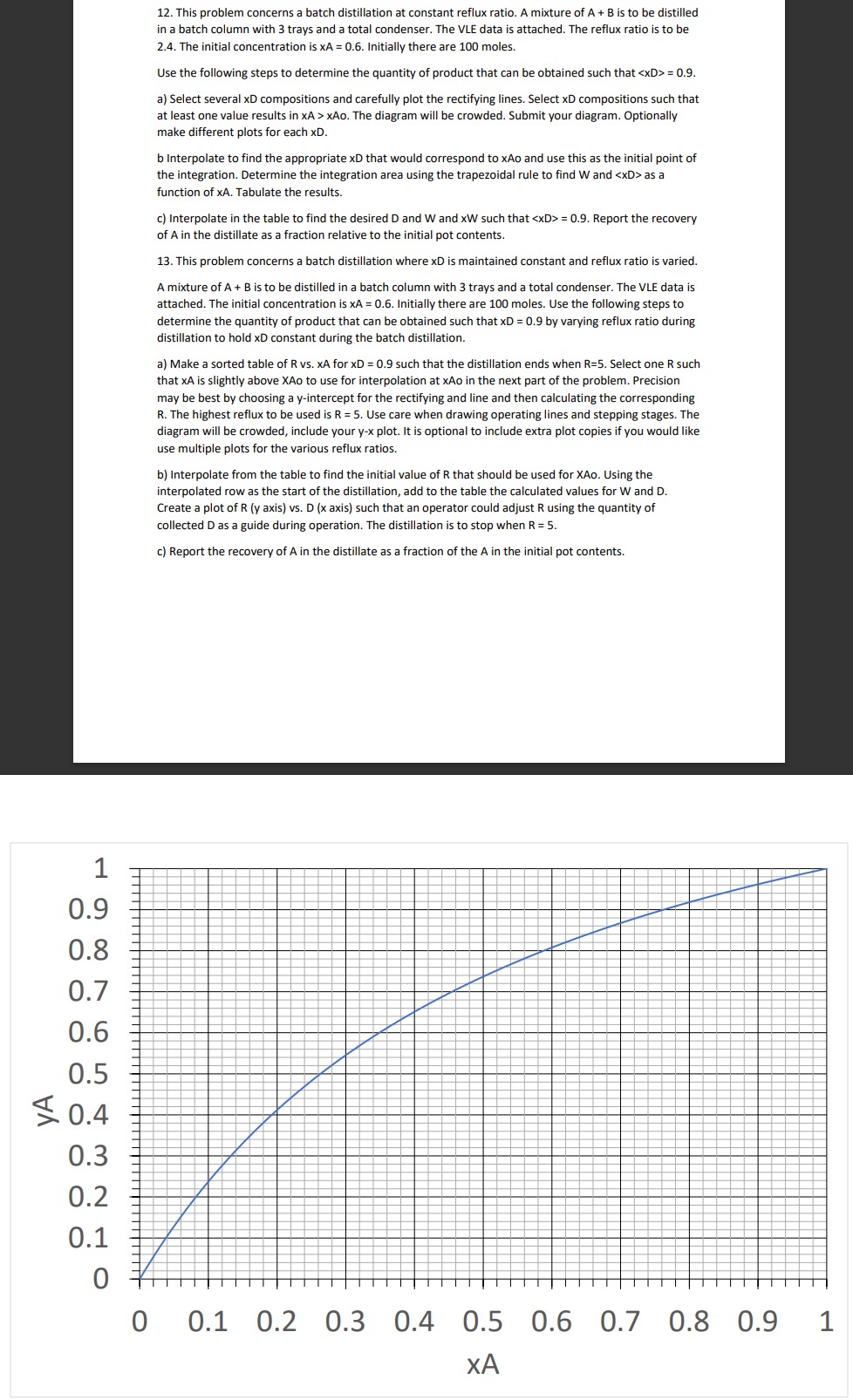
This problem concerns a batch distillation at constant reflux ratio. A mixture of A + B is to be distilled in a batch column with
This problem concerns a batch distillation at constant reflux ratio. A mixture of is to be distilled
in a batch column with trays and a total condenser. The VLE data is attached. The reflux ratio is to be
The initial concentration is Initially there are moles.
Use the following steps to determine the quantity of product that can be obtained such that ::
a Select several XD compositions and carefully plot the rectifying lines. Select XD compositions such that
at least one value results in The diagram will be crowded. Submit your diagram. Optionally
make different plots for each
b Interpolate to find the appropriate xD that would correspond to XAo and use this as the initial point of
the integration. Determine the integration area using the trapezoidal rule to find and as a
function of Tabulate the results.
c Interpolate in the table to find the desired and and such that :: Report the recovery
of in the distillate as a fraction relative to the initial pot contents.
This problem concerns a batch distillation where xD is maintained constant and reflux ratio is varied.
A mixture of is to be distilled in a batch column with trays and a total condenser. The VLE data is
attached. The initial concentration is Initially there are moles. Use the following steps to
determine the quantity of product that can be obtained such that by varying reflux ratio during
distillation to hold xD constant during the batch distillation.
a Make a sorted table of vs for such that the distillation ends when Select one such
that is slightly above XAo to use for interpolation at xAo in the next part of the problem. Precision
may be best by choosing a intercept for the rectifying and line and then calculating the corresponding
The highest reflux to be used is Use care when drawing operating lines and stepping stages. The
diagram will be crowded, include your x plot. It is optional to include extra plot copies if you would like
use multiple plots for the various reflux ratios.
b Interpolate from the table to find the initial value of R that should be used for XAo. Using the
interpolated row as the start of the distillation, add to the table the calculated values for W and D
Create a plot of y axis vsx axis such that an operator could adjust using the quantity of
collected as a guide during operation. The distillation is to stop when
c Report the recovery of in the distillate as a fraction of the in the initial pot contents.
Please answer this by drawing it out with the graph please
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


