Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(this problem has multiple steps) please help with all parts A catalyst decreases the activation energy of a particular exothermic reaction by 29 kJ/mol, to
(this problem has multiple steps) please help with all parts 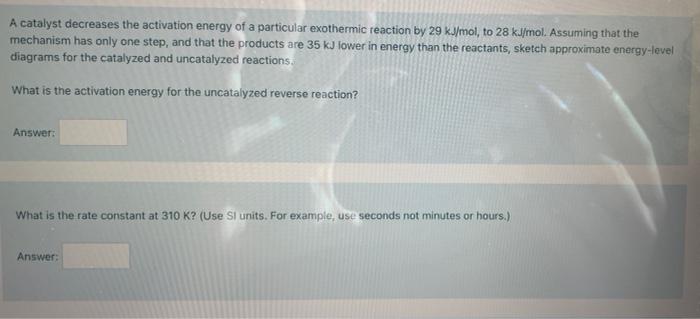
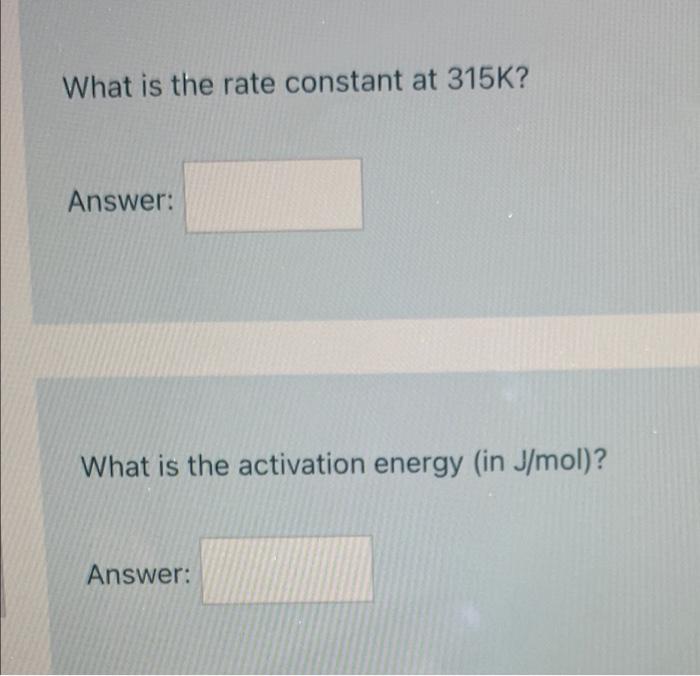
A catalyst decreases the activation energy of a particular exothermic reaction by 29 kJ/mol, to 28 kJ/mol. Assuming that the mechanism has only one step, and that the products are 35 kJ lower in energy than the reactants, sketch approximate energy-level diagrams for the catalyzed and uncatalyzed reactions What is the activation energy for the uncatalyzed reverse reaction? Answer: What is the rate constant at 310 K? (Use SI units. For example, use seconds not minutes or hours.) Answer: What is the rate constant at 315K? Answer: What is the activation energy in J/mol) 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


