Answered step by step
Verified Expert Solution
Question
1 Approved Answer
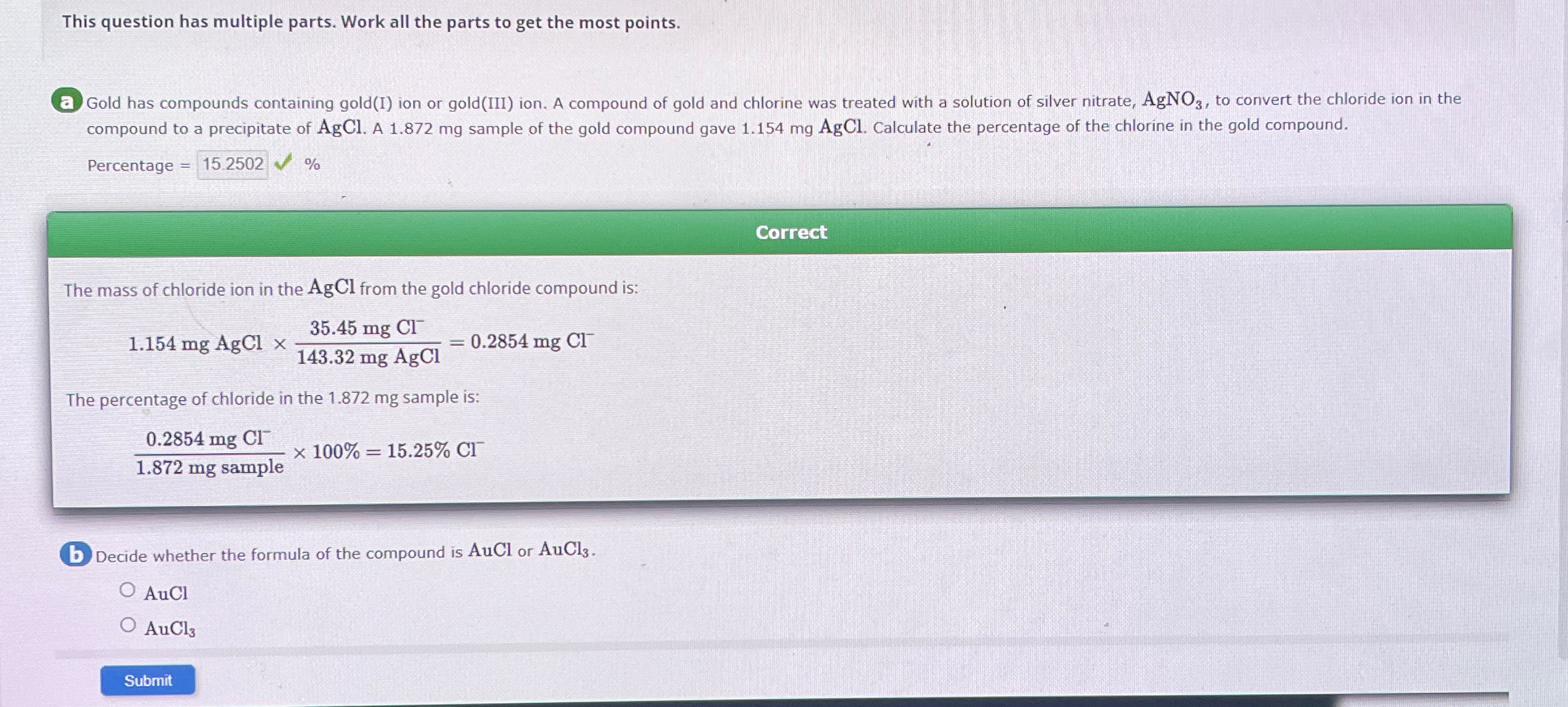
This question has multiple parts. Work all the parts to get the most points. Gold has compounds containing gold ( I ) ion or gold
This question has multiple parts. Work all the parts to get the most points.
Gold has compounds containing goldI ion or goldIII ion. A compound of gold and chlorine was treated with a solution of silver nitrate, to convert the chloride ion in the compound to a precipitate of AgCl. A sample of the gold compound gave mgAgCl. Calculate the percentage of the chlorine in the gold compound.
Percentage
Correct
The mass of chloride ion in the AgCl from the gold chloride compound is:
mgAgCl
The percentage of chloride in the sample is:
Decide whether the formula of the compound is AuCl or
AuCl
please answer part b only
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


