Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This textbook problem is solved k need it reevaluated wit the new degrees that i put right about the old one thank you. Example 8.5-2
This textbook problem is solved k need it reevaluated wit the new degrees that i put right about the old one thank you. 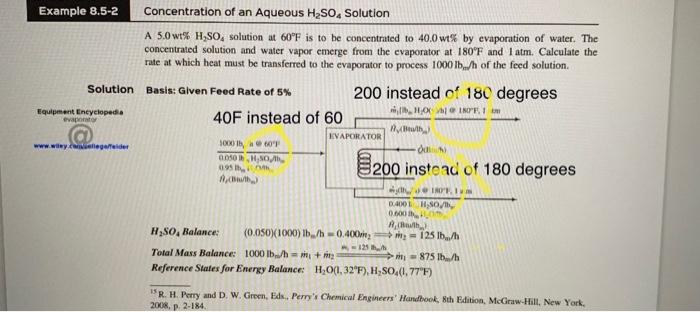
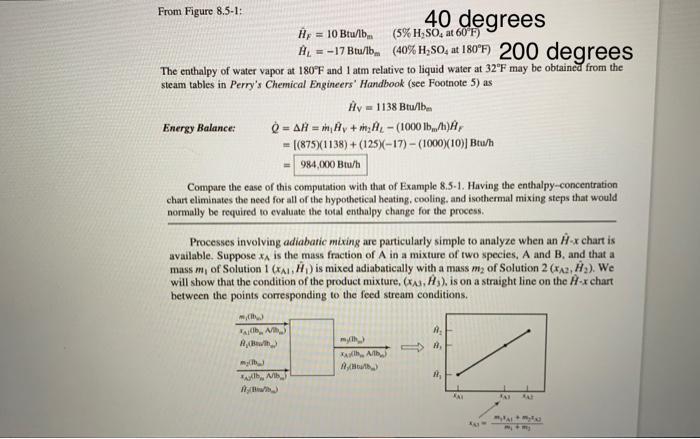
Example 8.5-2 Concentration of an Aqueous H2SO4 Solution A 5.0 wt% H2SO, solution at 60F is to be concentrated to 40.0 wt% by evaporation of water. The concentrated solution and water vapor emerge from the evaporator at 180F and I atm. Calculate the rate at which heat must be transferred to the evaporator to process 100015./h of the feed solution Solution Basis: Given Feed Rate of 5% 200 instead of 180 degrees , , 1 Equipment Encyclopedia 40F instead of 60 evapor Aunty KVAPORATOR 3000 www.wiley.collegareider 0.080 HS 09 8200 instead of 180 degrees AB INDI 0.001 / 0.000,00 Rw R250, Balance: (0.050)(1000) b/-0.400) = 125 lb/h = 125 Total Mass Balance 1000 lb/h = + 2 m = 875 lb/h Reference States for Energy Balance H20(1,32"F), H,SO.(1,77"F) WR H. Perry and D. W. Green, Eds.. Perry's Chemical Engineers' Handbook 8th Edition, McGraw-Hill, New York, 2008, p. 2-184 From Figure 8.5-1: 40 degrees Hy = 10 Btu/lb. (5% H2SO, at 60F) H. = -17 Btw(40% H80, at 180F) 200 degrees The enthalpy of water vapor at 180F and I atm relative to liquid water at 32F may be obtained from the steam tables in Perry's Chemical Engineers' Handbook (see Footnote 5) as Hv = 1138 Btu/ibm Energy Balance O = AH = Hy + m, HL - 1000 15/h)A, = [(875)(1138) + (125-17) - (1000)(10))Btu/h 984,000 Btu/h Compare the case of this computation with that of Example 8.5-1. Having the enthalpy-concentration chart eliminates the need for all of the hypothetical heating, cooling, and isothermal mixing steps that would normally be required to evaluate the total enthalpy change for the process. Processes involving adiabatic mixing are particularly simple to analyze when an ex chart is available. Suppose is the mass fraction of A in a mixture of two species. A and B, and that a mass m, of Solution 1 (XA1,) is mixed adiabatically with a mass my of Solution 2 (XA2, H2). We will show that the condition of the product mixture. (A,A), is on a straight line on the H-x chart between the points corresponding to the feed stream conditions. mich 3 RB) with Alib) Amala 11, Hisial RA AL 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


