Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Those clever chemists 1 are always coming up with interesting synthetic processes, such as this one: In this synthesis buckminster fullerene (C60, or buckyballs) is
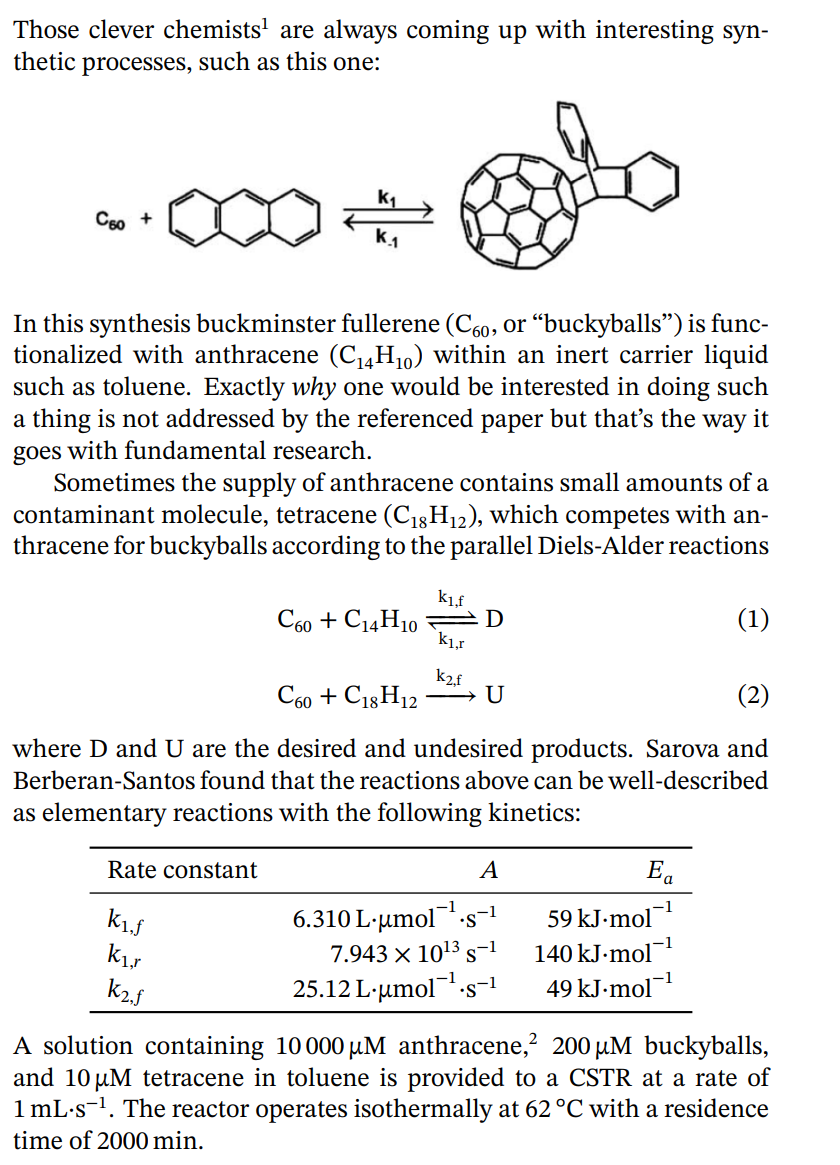

 Those clever chemists 1 are always coming up with interesting synthetic processes, such as this one: In this synthesis buckminster fullerene (C60, or "buckyballs") is functionalized with anthracene (C14H10) within an inert carrier liquid such as toluene. Exactly why one would be interested in doing such a thing is not addressed by the referenced paper but that's the way it goes with fundamental research. Sometimes the supply of anthracene contains small amounts of a contaminant molecule, tetracene (C18H12), which competes with anthracene for buckyballs according to the parallel Diels-Alder reactions C60+C14H10k1,rk1,fDC60+C18H12k2,fU where D and U are the desired and undesired products. Sarova and Berberan-Santos found that the reactions above can be well-described as elementary reactions with the following kinetics: A solution containing 10000M anthracene, 2200M buckyballs, and 10M tetracene in toluene is provided to a CSTR at a rate of 1mLs1. The reactor operates isothermally at 62C with a residence time of 2000min. 1 G.H. Sarova; M.N. Berberan-Santos. Kinetics of the Diels-Alder reaction between C60 and acenes. Chem. Phys. Lett. 397 (2004) 402407. 2The unit " M " is micromolar, or 1106molL1. Calculate the volume of the reactor and compare your answer to an everyday object
Those clever chemists 1 are always coming up with interesting synthetic processes, such as this one: In this synthesis buckminster fullerene (C60, or "buckyballs") is functionalized with anthracene (C14H10) within an inert carrier liquid such as toluene. Exactly why one would be interested in doing such a thing is not addressed by the referenced paper but that's the way it goes with fundamental research. Sometimes the supply of anthracene contains small amounts of a contaminant molecule, tetracene (C18H12), which competes with anthracene for buckyballs according to the parallel Diels-Alder reactions C60+C14H10k1,rk1,fDC60+C18H12k2,fU where D and U are the desired and undesired products. Sarova and Berberan-Santos found that the reactions above can be well-described as elementary reactions with the following kinetics: A solution containing 10000M anthracene, 2200M buckyballs, and 10M tetracene in toluene is provided to a CSTR at a rate of 1mLs1. The reactor operates isothermally at 62C with a residence time of 2000min. 1 G.H. Sarova; M.N. Berberan-Santos. Kinetics of the Diels-Alder reaction between C60 and acenes. Chem. Phys. Lett. 397 (2004) 402407. 2The unit " M " is micromolar, or 1106molL1. Calculate the volume of the reactor and compare your answer to an everyday object Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


