Answered step by step
Verified Expert Solution
Question
1 Approved Answer
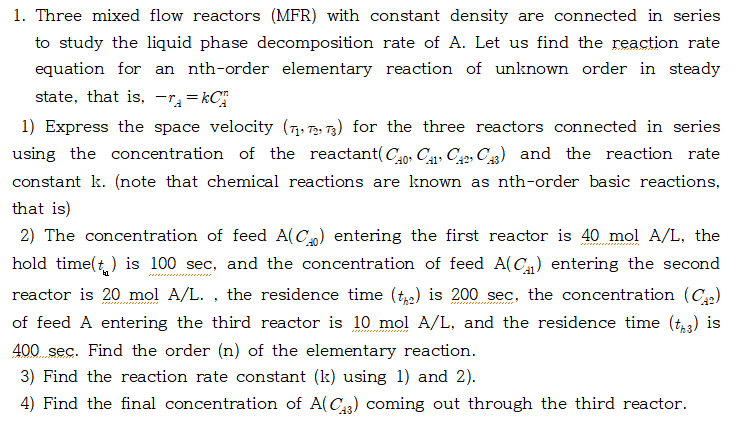
Three mixed flow reactors ( MFR ) with constant density are connected in series to study the liquid phase decomposition rate of A . Let
Three mixed flow reactors MFR with constant density are connected in series
to study the liquid phase decomposition rate of A Let us find the reaction rate
equation for an nthorder elementary reaction of unknown order in steady
state, that is
Express the space velocity for the three reactors connected in series
using the concentration of the reactant and the reaction rate
constant note that chemical reactions are known as nthorder basic reactions,
that is
The concentration of feed entering the first reactor is mol the
hold time is sec and the concentration of feed entering the second
reactor is mol the residence time is the concentration
of feed A entering the third reactor is mol and the residence time is
sec Find the order of the elementary reaction.
Find the reaction rate constant k using and
Find the final concentration of coming out through the third reactor.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


