Answered step by step
Verified Expert Solution
Question
1 Approved Answer
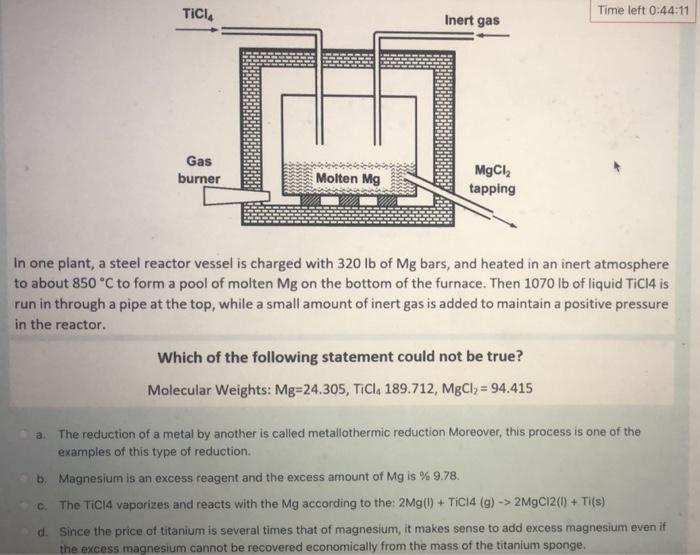
TICI4 Gas burner Molten Mg 5 Inert gas MgCl, tapping Time left 0:44:11 In one plant, a steel reactor vessel is charged with 320 lb
TICI4 Gas burner Molten Mg 5 Inert gas MgCl, tapping Time left 0:44:11 In one plant, a steel reactor vessel is charged with 320 lb of Mg bars, and heated in an inert atmosphere to about 850 C to form a pool of molten Mg on the bottom of the furnace. Then 1070 lb of liquid TiC14 is run in through a pipe at the top, while a small amount of inert gas is added to maintain a positive pressure in the reactor. Which of the following statement could not be true? Molecular Weights: Mg-24.305, TiCl4 189.712, MgCl = 94.415 a. The reduction of a metal by another is called metallothermic reduction Moreover, this process is one of the examples of this type of reduction. b. Magnesium is an excess reagent and the excess amount of Mg is % 9.78. c. The TiC14 vaporizes and reacts with the Mg according to the: 2Mg(1) + TiC14 (g) -> 2MgCl2(1) + Ti(s) d. Since the price of titanium is several times that of magnesium, it makes sense to add excess magnesium even if the excess magnesium cannot be recovered economically from the mass of the titanium sponge.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


