Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Time left 0:48:26 Question 1 Not yet answered Three compounds (A-C) yield the solubilities and chemical tests described below. Compound A insoluble in water, NaOH,
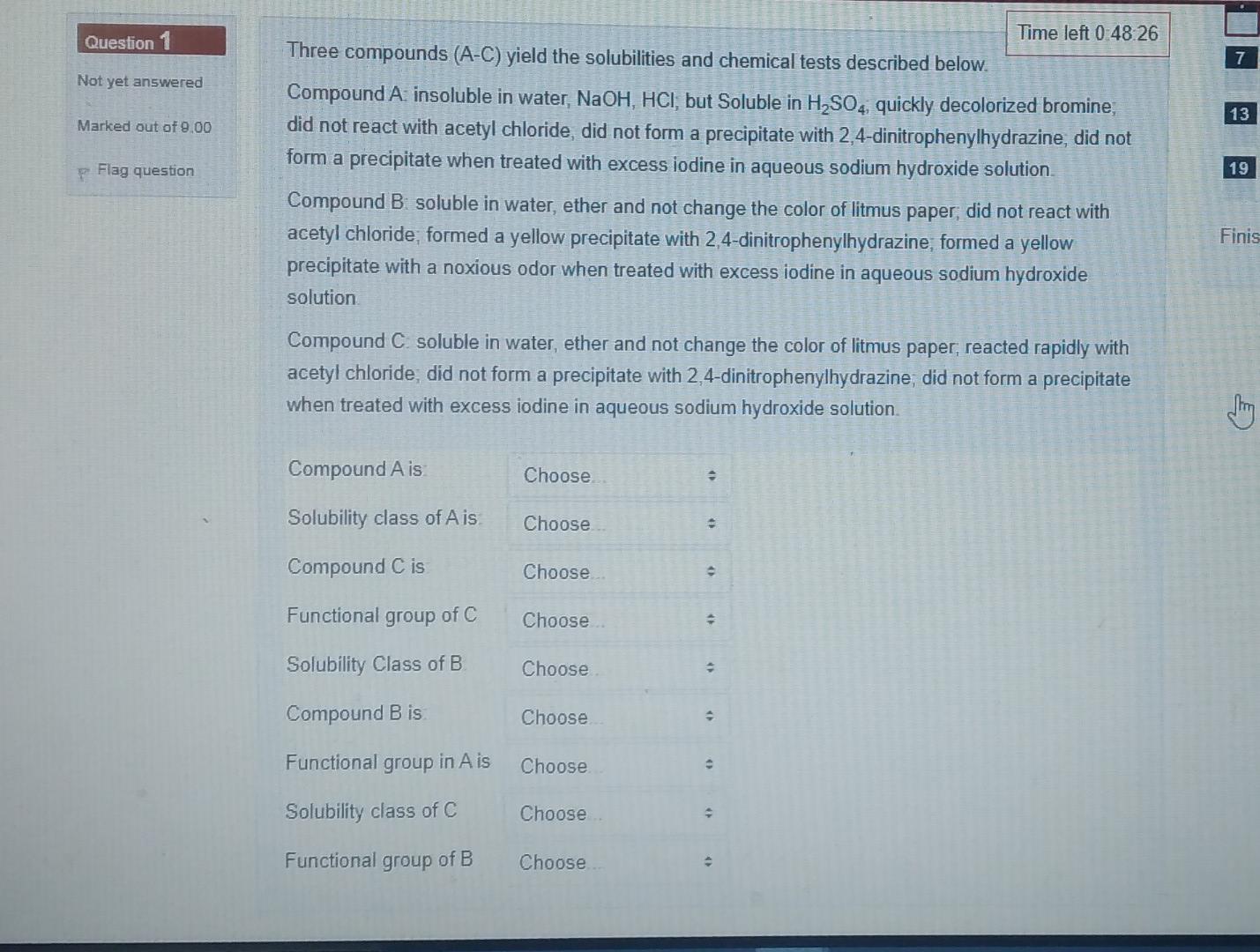
Time left 0:48:26 Question 1 Not yet answered Three compounds (A-C) yield the solubilities and chemical tests described below. Compound A insoluble in water, NaOH, HCI, but Soluble in H2SO4, quickly decolorized bromine, did not react with acetyl chloride, did not form a precipitate with 2,4-dinitrophenylhydrazine, did not form a precipitate when treated with excess iodine in aqueous sodium hydroxide solution 13 Marked out of 9.00 p Flag question 19 Finis Compound B. soluble in water, ether and not change the color of litmus paper, did not react with acetyl chloride, formed a yellow precipitate with 2,4-dinitrophenylhydrazine, formed a yellow precipitate with a noxious odor when treated with excess iodine in aqueous sodium hydroxide solution Compound C. soluble in water, ether and not change the color of litmus paper, reacted rapidly with acetyl chloride, did not form a precipitate with 2,4-dinitrophenylhydrazine, did not form a precipitate when treated with excess iodine in aqueous sodium hydroxide solution G Compound Ais Choose Solubility class of Ais Choose Compound C is Choose Functional group of C Choose Solubility Class of B Choose Compound B is Choose Functional group in A is Choose Solubility class of C Choose Functional group of B Choose
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


