Answered step by step
Verified Expert Solution
Question
1 Approved Answer
titration Name Answer each question below. 1. Calculate the concentration of a 25ml. NoOH solution if 30mL of 125MHCl is nceded to tht ate to
titration 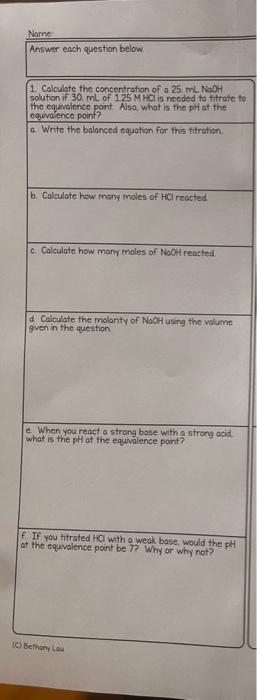
Name Answer each question below. 1. Calculate the concentration of a 25ml. NoOH solution if 30mL of 125MHCl is nceded to tht ate to the equavience point. Also, what is the pH at the equivalence point? a. Write the balanced equation for this titration. b. Colculate how many moles of HCl redcted. c. Calculate how many moles of NoOH reacted. d. Calculate the molanty of NaOH using the volume gven in the question e. When you react a strong base with a strong acid. what is the plt at the equivelence paint? f. If you titrated HOl with o weak base, would the pH at the equivalence point be 7?. Why or why not? (c) Bethani Los 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


