Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Titration of a polyprotic acid 4. Lisinopril is a drug used in the treatment of high blood pressure, congestive heart failure and diabetes. It works
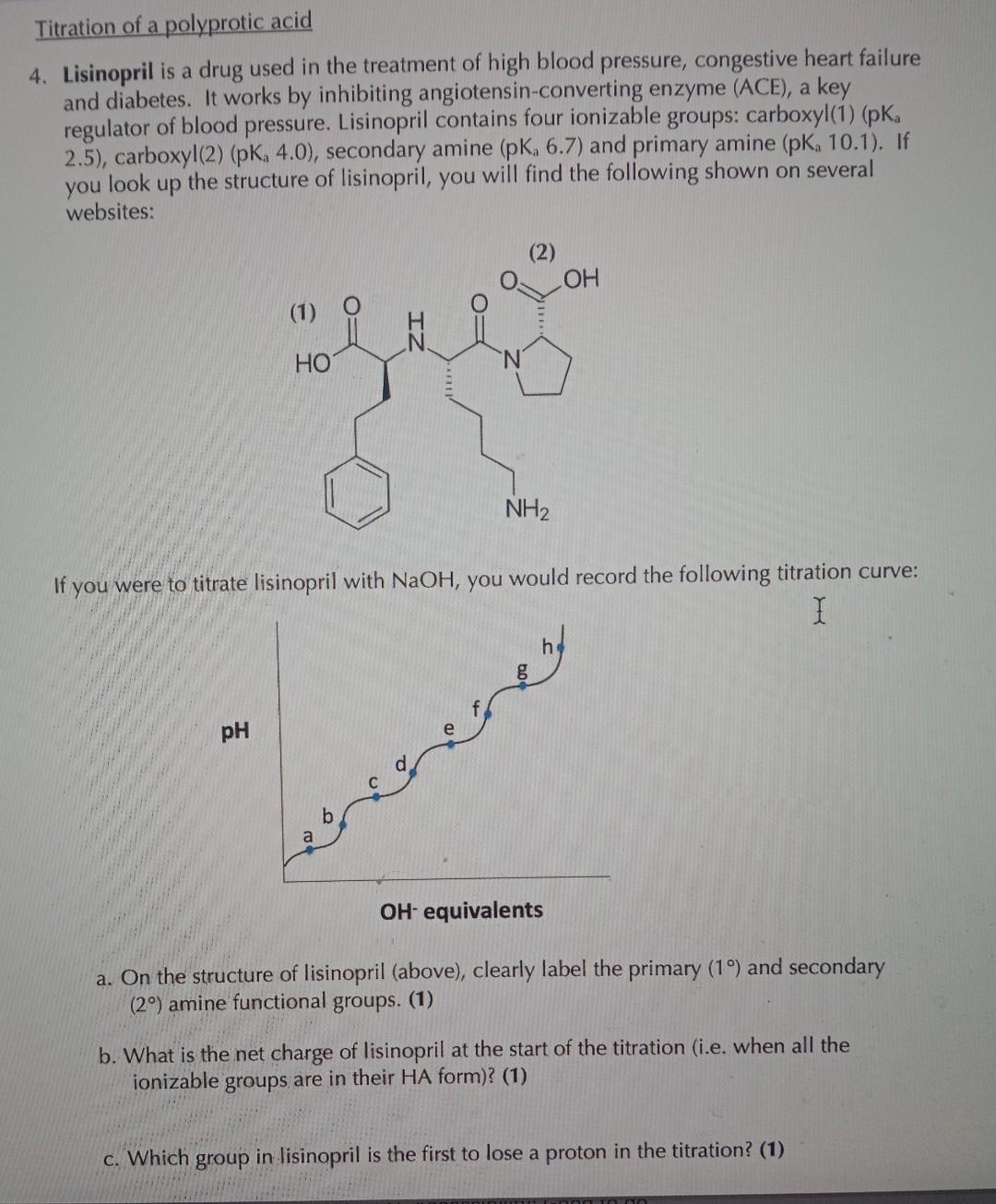
Titration of a polyprotic acid 4. Lisinopril is a drug used in the treatment of high blood pressure, congestive heart failure and diabetes. It works by inhibiting angiotensin-converting enzyme (ACE), a key regulator of blood pressure. Lisinopril contains four ionizable groups: carboxyl(1) (pKa 2.5), carboxyl(2) (pKa4.0), secondary amine (pKa 6.7) and primary amine (pKa10.1). If you look up the structure of lisinopril, you will find the following shown on several websites: If you were to titrate lisinopril with NaOH, you would record the following titration curve: a. On the structure of lisinopril (above), clearly label the primary (1) and secondary (2) amine functional groups. (1) b. What is the net charge of lisinopril at the start of the titration (i.e. when all the ionizable groups are in their HA form)? (1) c. Which group in lisinopril is the first to lose a proton in the titration? (1)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


