Question
To a 1.00 L solution of 0.35 M Na2SO4, you add 5 mL of 9.89 M Ca(NO3)2 to produce the insoluble CaSO4 precipitate as
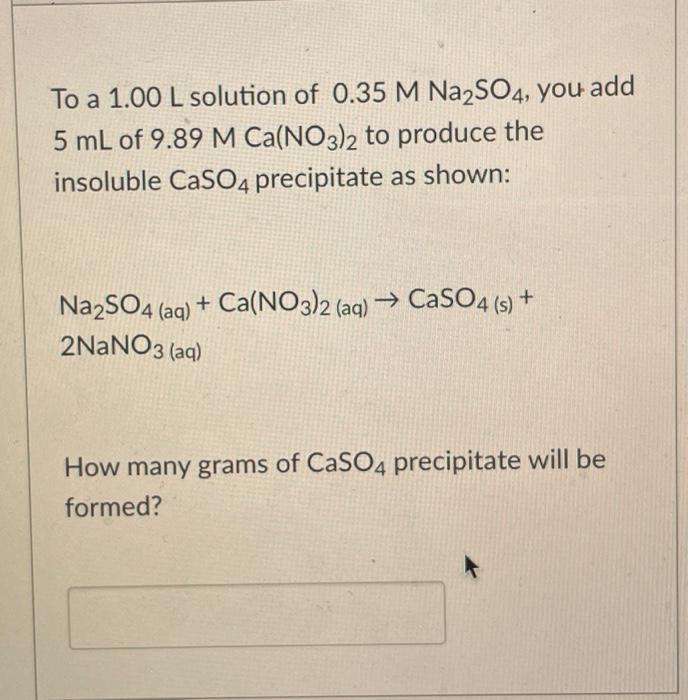
To a 1.00 L solution of 0.35 M Na2SO4, you add 5 mL of 9.89 M Ca(NO3)2 to produce the insoluble CaSO4 precipitate as shown: NazSO4 (aq) + Ca(NO3)2 (aq) CaSO4 (5) + 2NANO3 (aq) How many grams of CaSO4 precipitate will be formed?
Step by Step Solution
3.39 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Conceptual Physical Science
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
6th edition
013408229X, 978-0134082295, 9780134080512 , 978-0134060491
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



