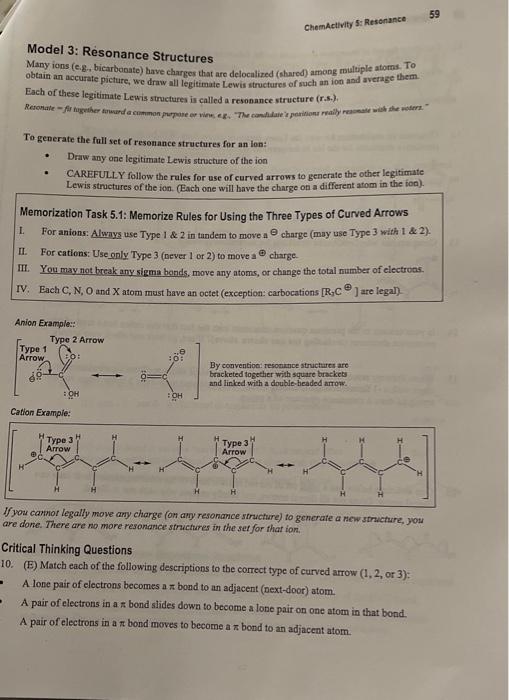
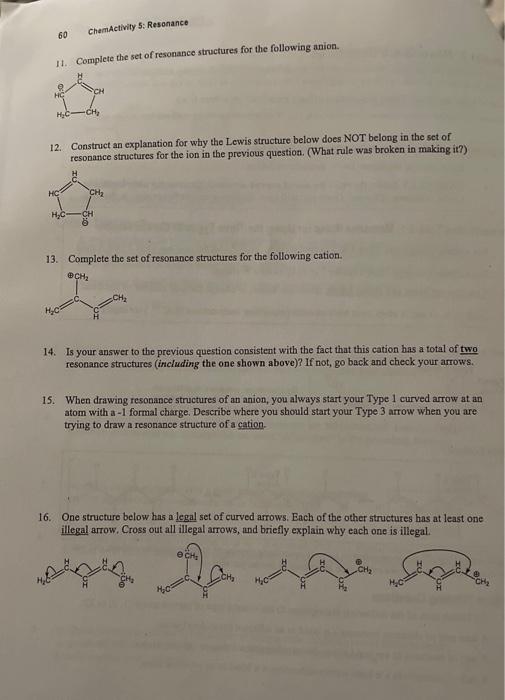
To generate the full set of resonance struefures for an ion: - Diaw any one legitimate Lewis structure of the ion - CAREFULLY follow the rules for ase of curved arrows to generate the other legitimate Lewis structures of the ion. (Each one will have the charge on a different atom in the ion). Memorization Task 5.1: Memorize Rules for Using the Three Types of Curved Arrows L. For anions: Alwnys use Type 1&2 in tandem to move a charge (may use Type 3 with 1 \& 2). II. For cations: Use only Type 3 (never 1 or 2) to move a charge. III. You may not boeak any sigma bonds, move any atoms, or change the total number of electrons. IV. Each C,N,O and X atom must bave an octet (exception: carbocations [R3C9 ] are legal)- Anion Examples. By coavention: resoninde structures are bracketed together with square brackets and linked with a double-headed arrow. Cation Example: If you cannor legally move any charge (on apy resonance struchure) to generate a new structure, you are done. There are no more resonance strictures in the set for that ion, Critical Thinking Questions 10. (E) Match each of the following descriptions to the correct type of curved arrow (1,2, or 3); A lone pair of electrons becomes a bond to an adjacent (next-door) atom. A pair of electrons in a z bond slides down to become a lone pair on one atom in that bond. A pair of electrons in a bond moves to become a bond to an adjacent atom. 11. Complete the set of resonance structures for the following anion. 12. Construct an explanation for wiy the Lewis structure below does NOT belong in the set of resonance stnuctures for the ion in the previous question. (What rule was broken in making it?) 13. Complete the set of resonance structures for the following cation. 14. Is your answer to the previous question consistent with the fact that this cation has a total of two resonance structures (including the one shown above)? If not, go back and check your arrows. 15. When drawing resonance structures of an anion, you always start your Type 1 curved arrow at an atom with a -1 formal charge. Describe where you should start your Type 3 arrow when you are trying to draw a resonance structure of a cation. 16. One structure below has a legal set of curved arrows. Each of the other structures has at least one illegal arrow, Cross out all illegal arrows, and briefly explain why each one is illegal