Question
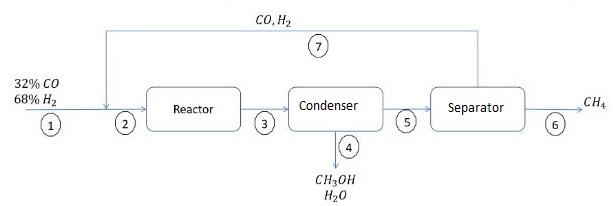
To produce methyl alcohol, the chemical reaction represented by the equation CO + 2H = CHOH is used. This is the main reaction because a
To produce methyl alcohol, the chemical reaction represented by the equation CO + 2H = CHOH is used.
This is the main reaction because a side reaction occurs represented by: CO + 3H=CH + HO.
The CO conversion per pass is 12.5%. Of this amount, 87.5% corresponds to the main reaction and the rest to the collateral reaction. The reactor outlet stream passes through a condenser where a liquid mixture of methanol and water is obtained, while the remaining gases are passed to a separator where all the methane is removed and the other gases are recirculated. Calculate:
(a) Degrees of freedom for: the total process, each unit and the mixing point.
(b) The mass composition of the methanol-water mixture.
(c) The recycle ratio (moles of recycle/mole of fresh feed).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


