Answered step by step
Verified Expert Solution
Question
1 Approved Answer
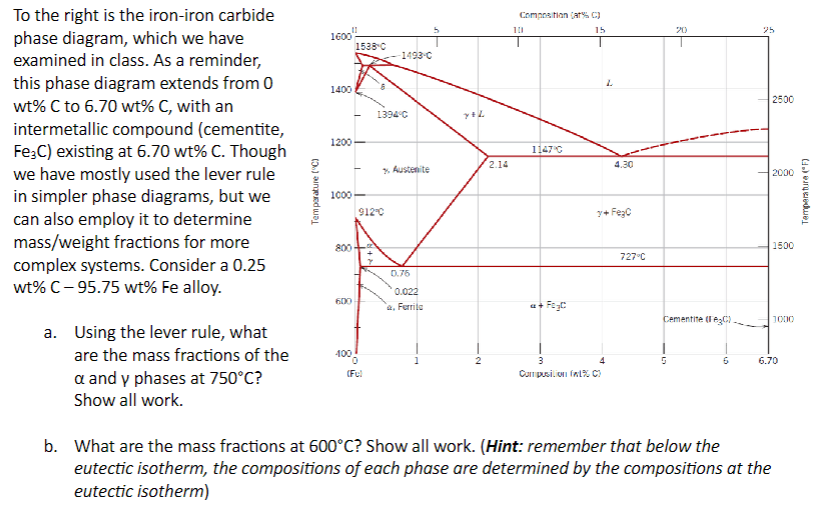
To the right is the iron - iron carbide phase diagram, which we have examined in class. As a reminder, this phase diagram extends from
To the right is the ironiron carbide
phase diagram, which we have
examined in class. As a reminder,
this phase diagram extends from
wt C to wt C with an
intermetallic compound cementite
existing at Though
we have mostly used the lever rule
in simpler phase diagrams, but we
can also employ it to determine
massweight fractions for more
complex systems. Consider a
wt C wt Fe alloy.
a Using the lever rule, what
are the mass fractions of the
and phases at
Show all work.
b What are the mass fractions at Show all work. Hint: remember that below the
eutectic isotherm, the compositions of each phase are determined by the compositions at the
eutectic isotherm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


