Answered step by step
Verified Expert Solution
Question
1 Approved Answer
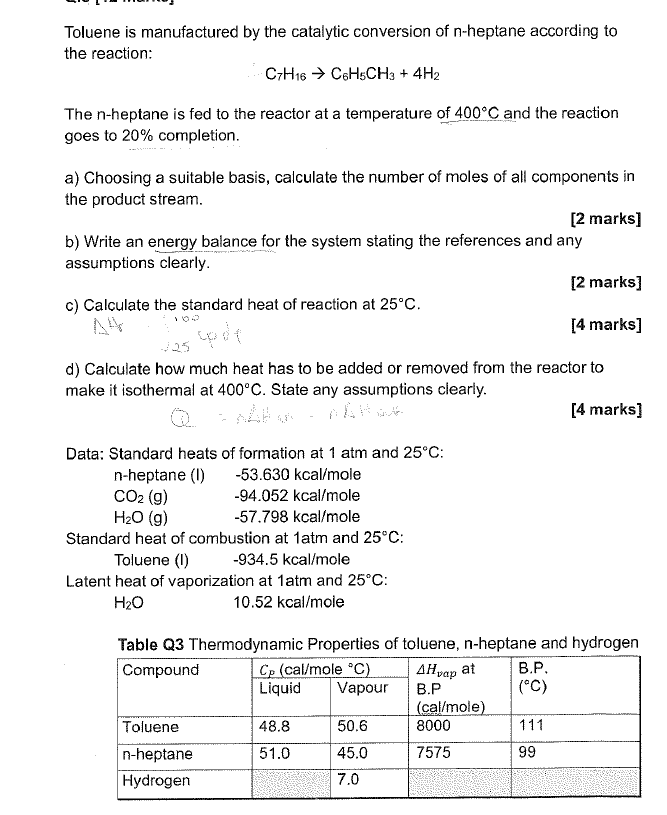
Toluene is manufactured by the catalytic conversion of n - heptane according to the reaction: C 7 H 1 6 C 6 H 5 C
Toluene is manufactured by the catalytic conversion of heptane according to
the reaction:
The heptane is fed to the reactor at a temperature of and the reaction
goes to completion.
a Choosing a suitable basis, calculate the number of moles of all components in
the product stream.
marks
b Write an energy balance for the system stating the references and any
assumptions clearly.
marks
c Calculate the standard heat of reaction at
marks
d Calculate how much heat has to be added or removed from the reactor to
make it isothermal at State any assumptions clearly.
marks
Data: Standard heats of formation at atm and :
nheptane Ikcaole
kcaole
kcaole
Standard heat of combustion at atm and :
Toluene Ikcaole
Latent heat of vaporization at atm and :
kcaole
Table Q Thermodynamic Properties of toluene, nheptane and hydrogen
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


