Question
Toluene reacts with H 2 to form benzene (B), but a side reaction occurs in which a by-product diphenyl (D) is formed: The process is
Toluene reacts with H2 to form benzene (B), but a side reaction occurs in which a by-product diphenyl (D) is formed:
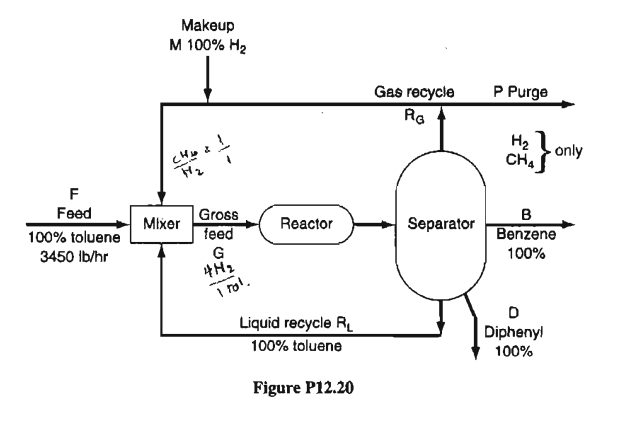
The process is shown in Figure P12.20. Hydrogen is added to the gas recycle stream to make the ratio of H2 to CH4 1 to 1 before the gas enters the mixer. The ratio of H2 to toluene entering the reactor at G is 4H2 to 1 toluene. The conversion of toluene to benzene on one pass through the reactor is 80%, and the conversion of toluene to by-product diphenyl is 8% on the same pass. Calculate the moles of RG, and moles of RL, per hour.
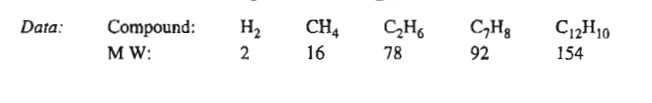
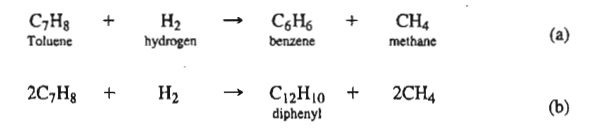
 TolueneC7H8+hydrogenH2benzeneC6H6+methaneCH42C7H8+H2C12H10+2CH4dipheny
TolueneC7H8+hydrogenH2benzeneC6H6+methaneCH42C7H8+H2C12H10+2CH4dipheny Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


