Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Transcribed: H2(g) + Cl2(g) -> 2HCl(g) The table to the right gives data for a reaction rate study of the reaction represented above. The reaction
Transcribed: 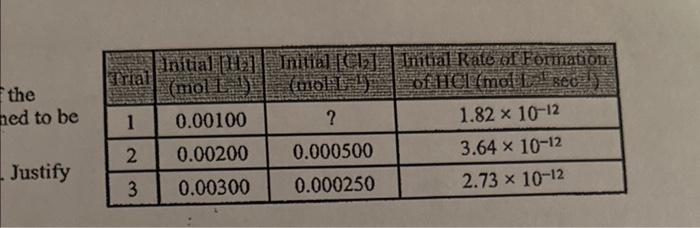
The table to the right gives data for a reection rate stady of the reaction represented above. The reaction has been determie ed to be first order with respect to Cl2. 9. 1 . 4 . Determine the arder of the reaction with respect to Hs. Jastify your answer. 16. Calculate the rate constant for this reaction and include the units of the k. 11. What wotil be the concentration of Cl2 at the start of trial 1? 22. In trial 3 , what is the initial rate of change of H2 ? The gas-phave formation of nitrogen dioxide has the following two-itep mechanism. 2NON2+2O2N2+O22NO2fartatep.slowstep 3. 3h. Write the balanced equation for the overall reaction. /4. (s) Is the nitrogen atom, N2, a catalyst for the reaction or is it an intermediatet Explain. 5. Ws Assuming the balancing coeffients match the order for a given reactant, write the rate lawiequation for this reaction. the ned to be Justify H2(g) + Cl2(g) -> 2HCl(g)
The table to the right gives data for a reaction rate study of the reaction represented above. The reaction has been determined to be first order with respect to Cl2.
9. Determine the order of the reaction with respect to H2. Justify your answer.
10. Calculate the rate constant for this reaction and include the units of the k.
11. What woulr be the concentration of Cl2 at the start of trial 1?
12. In trial 3, what is the initial rate of change of H2?
The gas-phase formation of nitrogen dioxide has the following two-step mechanism.
2NO -> N2 + O2
N2 + 2O2 -> 2NO2
13. Write the balanced equation for the overall reaction.
14. Is the nitrogen atom, N2, a catalyst for the reaction or is it an intermediate? Explain.
15. Assuming the balancing coeffcients match the order for a given reactant, write the rate law/equation for this reaction 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


