Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Trans-[PtCl,Br2l2 belongs to the D2h point group. Its Pt-Cl symmetric IR stretch has A, symmetry. Its Pt-Cl asymmetric stretch as B1u symmetry. Use the
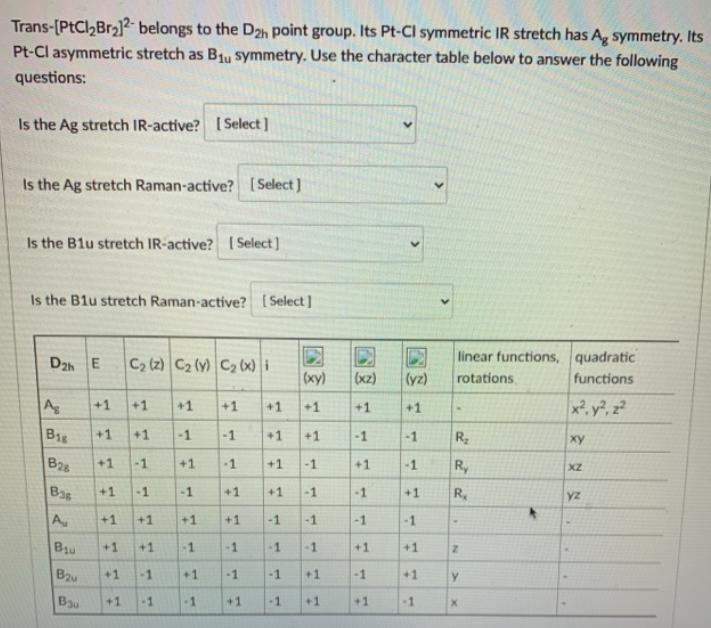
Trans-[PtCl,Br2l2 belongs to the D2h point group. Its Pt-Cl symmetric IR stretch has A, symmetry. Its Pt-Cl asymmetric stretch as B1u symmetry. Use the character table below to answer the following questions: Is the Ag stretch IR-active? [ Select] Is the Ag stretch Raman-active? [Select ) Is the Blu stretch IR-active? ( Select] Is the B1u stretch Raman-active? [Select] D2h E C2 (z) C2 (y) C2 (x) i linear functions, quadratic (xy) (xz) (yz) rotations functions A +1 +1 +1 +1 +1 +1 x, y, z2 +1 +1 B18 +1 +1 -1 -1 +1 +1 -1 -1 R B28 +1 -1 +1 -1 +1 -1 +1 -1 Ry XZ Bas -1 +1 -1 +1 +1 -1 -1 +1 R. yz A +1 +1 +1 +1 -1 -1 -1 -1 Bu +1 +1 -1 -1 -1 -1 +1 +1 B +1 -1 +1 -1 -1 +1 -1 +1 Bau +1 -1 -1 +1 -1 +1 +1 -1
Step by Step Solution
★★★★★
3.52 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Trans PtC12Br2 has centre of symmetryAccording to mutual exclusion principle the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


