Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Trichloroethylene, a widely used degreasing solvent for machine parts, is produced in a two-step reaction sequence. Ethylene is first chlorinated to yield tetrachloroethane, which is

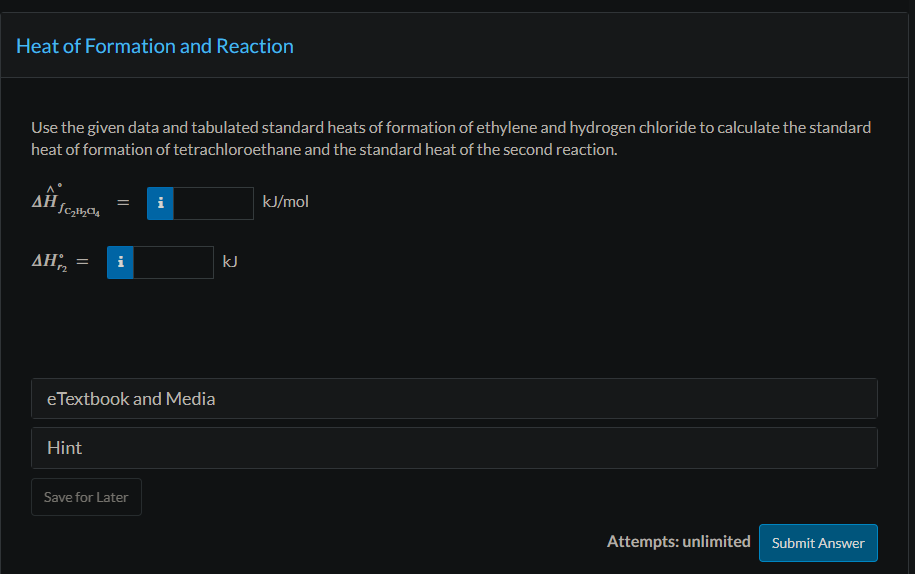
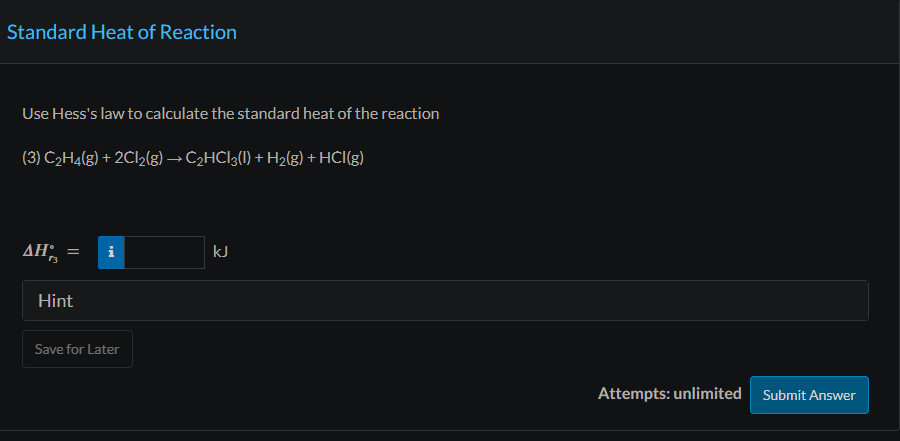
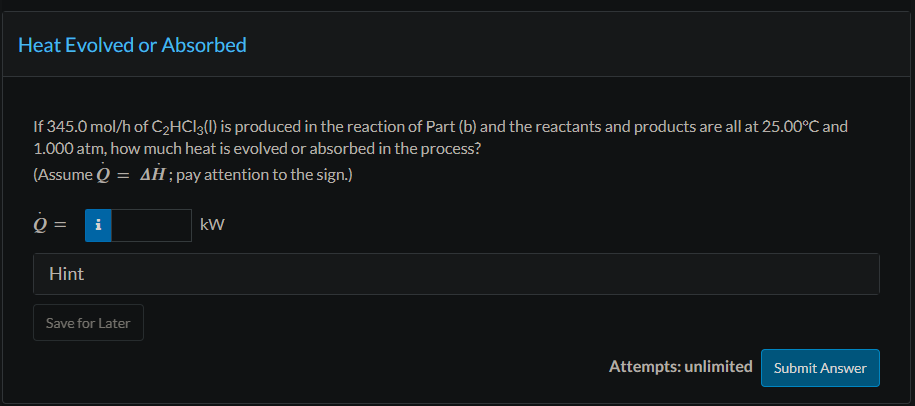 Trichloroethylene, a widely used degreasing solvent for machine parts, is produced in a two-step reaction sequence. Ethylene is first chlorinated to yield tetrachloroethane, which is dehydrochlorinated to form trichloroethylene. (1) C2H4(g)+2Cl2C2H2Cl4(l)+H2(g)Hr1=385.76kJ (2) C2H2Cl4(l)C2HCl3(l)+HCl(g) The standard heat of formation of liquid trichloroethylene is 276.2kJ/mol. Physical Property Tables Use the given data and tabulated standard heats of formation of ethylene and hydrogen chloride to calculate the standard heat of formation of tetrachloroethane and the standard heat of the second reaction. H^fC2H2C4=kJ/mol Hr2= Use Hess's law to calculate the standard heat of the reaction (3) C2H4(g)+2Cl2(g)C2HCl3(l)+H2(g)+HCl(g) If 345.0mol/h of C2HCl3(l) is produced in the reaction of Part (b) and the reactants and products are all at 25.00C and 1.000 atm, how much heat is evolved or absorbed in the process? (Assume Q=H; pay attention to the sign.)
Trichloroethylene, a widely used degreasing solvent for machine parts, is produced in a two-step reaction sequence. Ethylene is first chlorinated to yield tetrachloroethane, which is dehydrochlorinated to form trichloroethylene. (1) C2H4(g)+2Cl2C2H2Cl4(l)+H2(g)Hr1=385.76kJ (2) C2H2Cl4(l)C2HCl3(l)+HCl(g) The standard heat of formation of liquid trichloroethylene is 276.2kJ/mol. Physical Property Tables Use the given data and tabulated standard heats of formation of ethylene and hydrogen chloride to calculate the standard heat of formation of tetrachloroethane and the standard heat of the second reaction. H^fC2H2C4=kJ/mol Hr2= Use Hess's law to calculate the standard heat of the reaction (3) C2H4(g)+2Cl2(g)C2HCl3(l)+H2(g)+HCl(g) If 345.0mol/h of C2HCl3(l) is produced in the reaction of Part (b) and the reactants and products are all at 25.00C and 1.000 atm, how much heat is evolved or absorbed in the process? (Assume Q=H; pay attention to the sign.) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


