Answered step by step
Verified Expert Solution
Question
1 Approved Answer
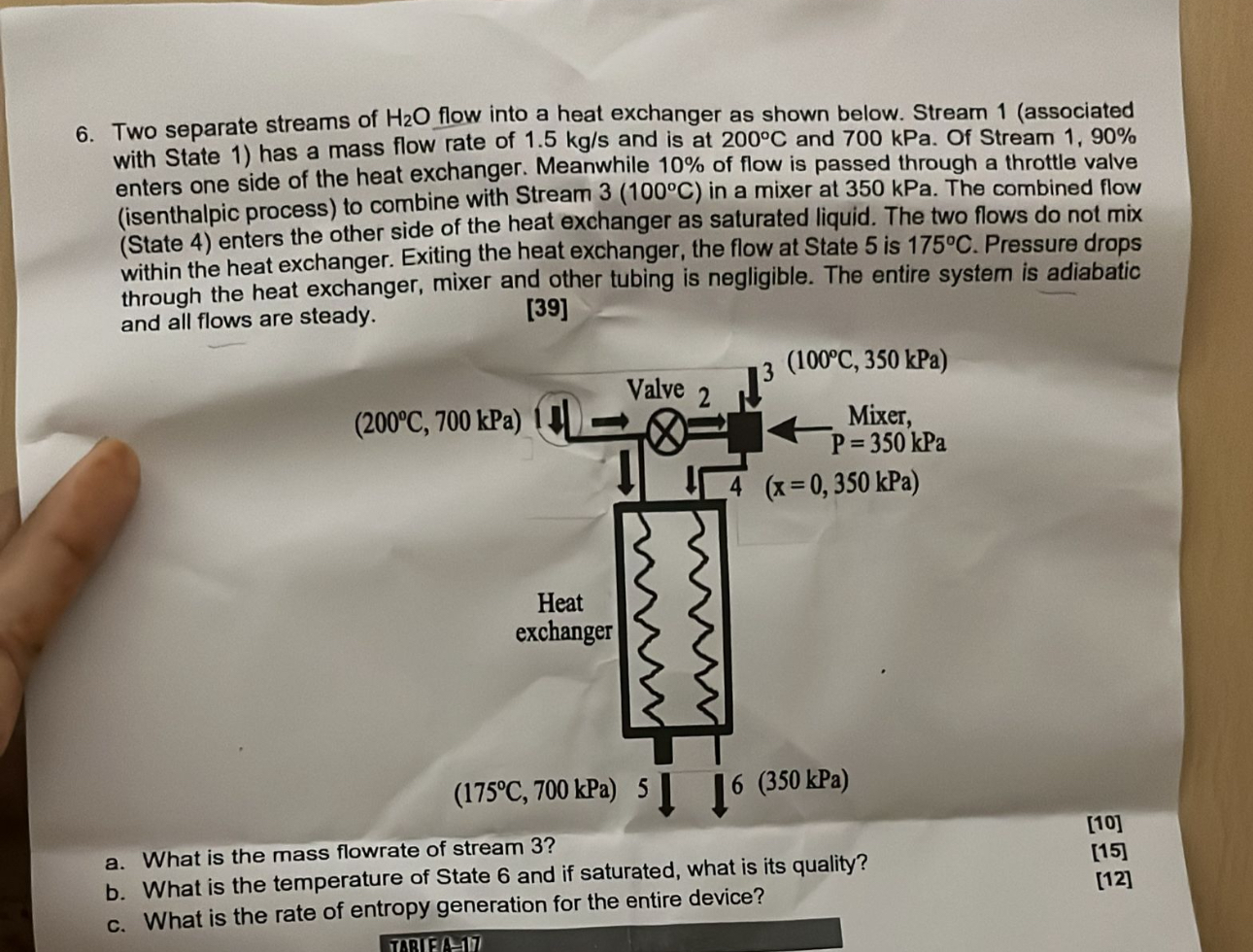
Two separate streams of H 2 O flow into a heat exchanger as shown below. Stream 1 ( associated with State 1 ) has a
Two separate streams of flow into a heat exchanger as shown below. Stream associated with State has a mass flow rate of and is at and kPa. Of Stream enters one side of the heat exchanger. Meanwhile of flow is passed through a throttle valve isenthalpic process to combine with Stream in a mixer at kPa. The combined flow State enters the other side of the heat exchanger as saturated liquid. The two flows do not mix within the heat exchanger. Exiting the heat exchanger, the flow at State is Pressure drops through the heat exchanger, mixer and other tubing is negligible. The entire system is adiabatic and all flows are steady.
a What is the mass flowrate of stream
b What is the temperature of State and if saturated, what is its quality?
c What is the rate of entropy generation for the entire device?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


