Question
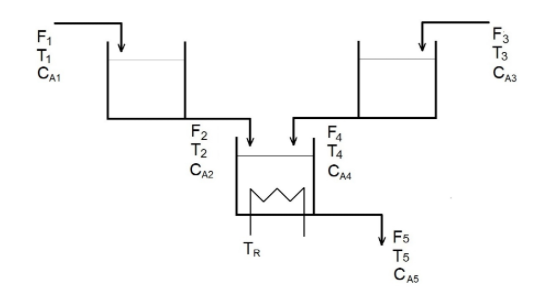
Two stirred tanks and a CSTR reactor with volumes equal to 13.26 ft3 each are used for produce compound B in an exothermic reaction A
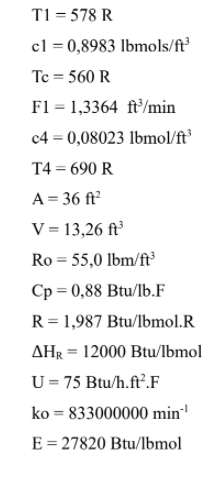
Two stirred tanks and a CSTR reactor with volumes equal to 13.26 ft3 each are used for produce compound B in an exothermic reaction A B with a final CA5 concentration of 0.08023 lbmols/ft3 at a constant flow rate of F1 and F3 = 1.3364 ft3/min, specific mass of 55.0lbm/ft3 and Cp = 0.88 Btu/lb.oF. The process is initially operating with the concentration of CA1 equal to 0.8983 lbmol/ft3 and CA3 = 0. Further system data is provided below.
Using Transfer Functions obtain the results:
(a) If the temperature of the refrigerant TR is increased by 10 Rankine what will be the new outlet temperature T5.
(b) If the inlet temperature T1 is suddenly increased from 578 to 588 Rankine, how long will it take for the temperature of
output T5 change 10 R?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


