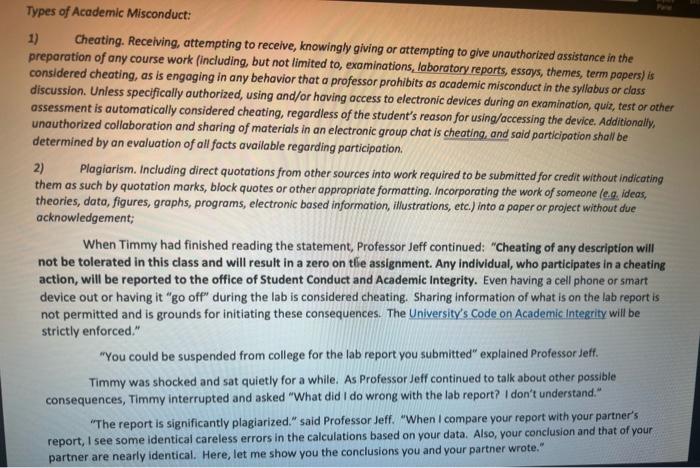
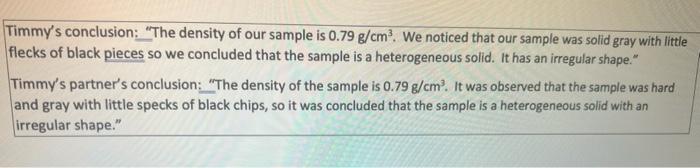
Types of Academic Misconduct: 1) Cheating. Receiving, attempting to receive, knowingly giving or attempting to give unauthorized assistance in the preparation of any course work (including, but not limited to, examinations, laboratory reports, essays, themes, term papers) is considered cheating, as is engaging in any behavior that a professor prohibits as academic misconduct in the syllabus or class discussion. Unless specifically authorized, using and/or having access to electronic devices during an examination, quiz, test or other assessment is automatically considered cheating, regardless of the student's reason for using/accessing the device. Additionally, unauthorized collaboration and sharing of materials in an electronic group chat is cheating, and said participation shall be determined by an evaluation of all facts available regarding participation 2) Plagiarism. Including direct quotations from other sources into work required to be submitted for credit without indicating them as such by quotation marks, block quotes or other appropriate formatting. Incorporating the work of someone le... ideas, theories, data, figures, graphs, programs, electronic based information, illustrations, etc.) into a paper or project without due acknowledgement; When Timmy had finished reading the statement, Professor Jeff continued: "Cheating of any description will not be tolerated in this class and will result in a zero on tle assignment. Any individual, who participates in a cheating action, will be reported to the office of Student Conduct and Academic Integrity. Even having a cell phone or smart device out or having it "go off" during the lab is considered cheating. Sharing information of what is on the lab report is not permitted and is grounds for initiating these consequences. The University's Code on Academic Integrity will be strictly enforced." "You could be suspended from college for the lab report you submitted" explained Professor Jeff Timmy was shocked and sat quietly for a while. As Professor Jeff continued to talk about other possible consequences, Timmy interrupted and asked "What did I do wrong with the lab report? I don't understand." "The report is significantly plagiarized." said Professor Jeff. "When I compare your report with your partner's report, I see some identical careless errors in the calculations based on your data. Also, your conclusion and that of your partner are nearly identical. Here, let me show you the conclusions you and your partner wrote." Timmy's conclusion: "The density of our sample is 0.79 g/cm? We noticed that our sample was solid gray with little flecks of black pieces so we concluded that the sample is a heterogeneous solid. It has an irregular shape." Timmy's partner's conclusion: "The density of the sample is 0.79 g/cm'. It was observed that the sample was hard and gray with little specks of black chips, so it was concluded that the sample is a heterogeneous solid with an irregular shape." Discuss and answer the following questions: 1) What about Timmy's actions are unethical? (5 points) 2) What could Timmy have done when writing the lab report to prevent the plagiarism? (5 points) 1 3) What is your definition of plagiarism? (5 points) 4) What actions could Jim suggest to resolve the problem as it now exists? (5 points) 5) How do the resolutions described in Question 4 affect the other students in the class? Provide a discussion of the fairness of such actions. (5 points)