Answered step by step
Verified Expert Solution
Question
1 Approved Answer
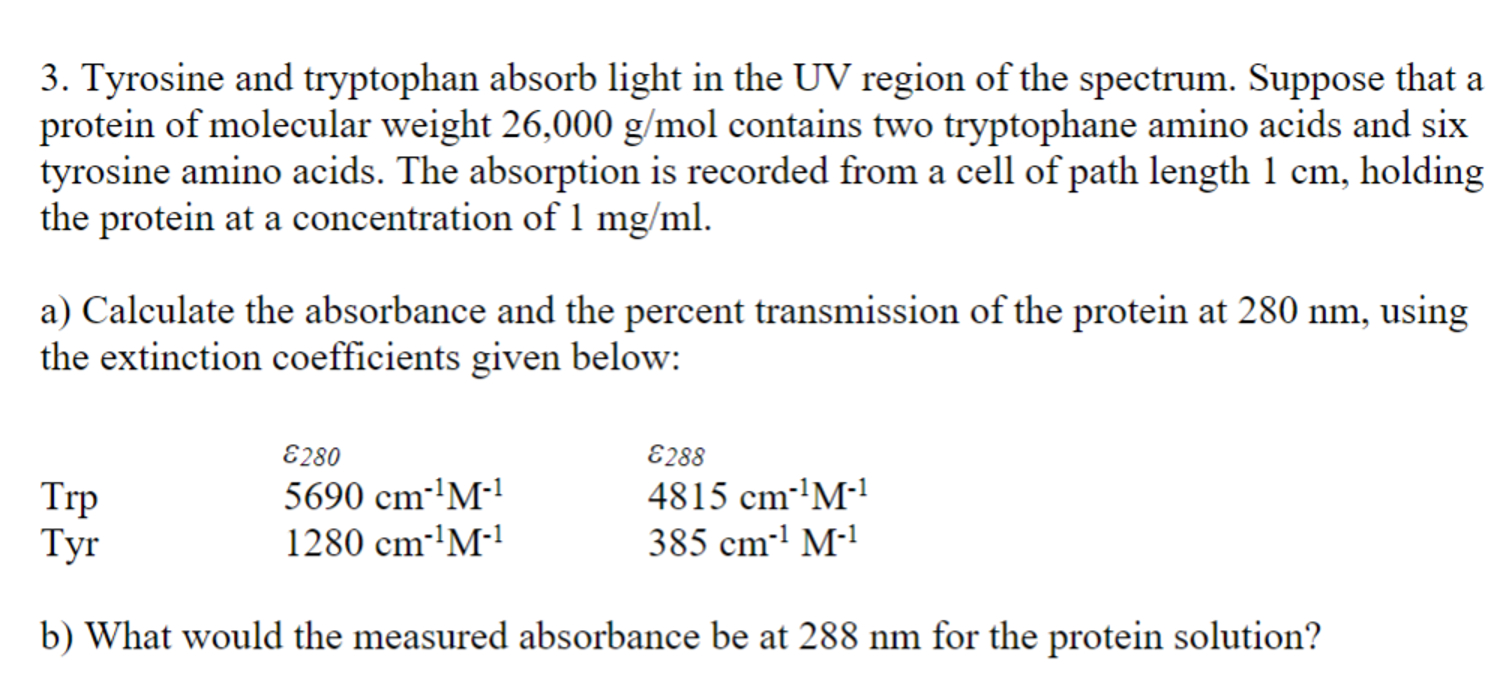
Tyrosine and tryptophan absorb light in the UV region of the spectrum. Suppose that a protein of molecular weight 2 6 , 0 0 0
Tyrosine and tryptophan absorb light in the UV region of the spectrum. Suppose that a
protein of molecular weight contains two tryptophane amino acids and six
tyrosine amino acids. The absorption is recorded from a cell of path length holding
the protein at a concentration of
a Calculate the absorbance and the percent transmission of the protein at using
the extinction coefficients given below:
b What would the measured absorbance be at for the protein solution?Tyrosine and tryptophan absorb light in the UV region of the spectrum. Suppose that a
protein of molecular weight gmol contains two tryptophane amino acids and six
tyrosine amino acids. The absorption is recorded from a cell of path length cm holding
the protein at a concentration of mgml
a Calculate the absorbance and the percent transmission of the protein at nm using
the extinction coefficients given below:
epsi epsi
Trp cm M cm M
Tyr cm M cm M
b What would the measured absorbance be at nm for the protein solution?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


