Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Urea (NH4CO), a common industrial fertilizer, is produced from ammonia (NH3) and carbon dioxide (CO). The reaction is given by 2NH3 + CO NH4CO
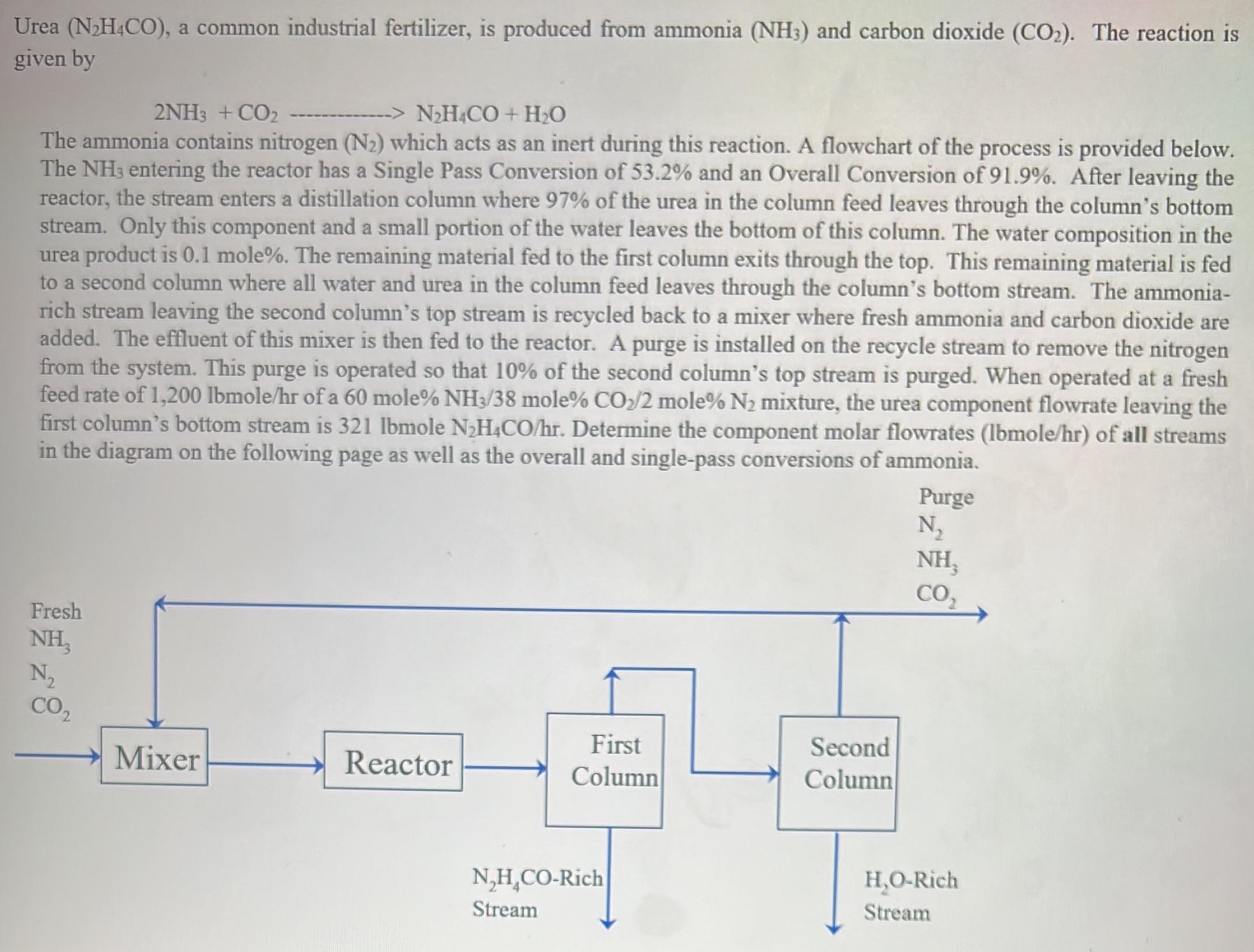
Urea (NH4CO), a common industrial fertilizer, is produced from ammonia (NH3) and carbon dioxide (CO). The reaction is given by 2NH3 + CO NH4CO + HO The ammonia contains nitrogen (N) which acts as an inert during this reaction. A flowchart of the process is provided below. The NH3 entering the reactor has a Single Pass Conversion of 53.2% and an Overall Conversion of 91.9%. After leaving the reactor, the stream enters a distillation column where 97% of the urea in the column feed leaves through the column's bottom stream. Only this component and a small portion of the water leaves the bottom of this column. The water composition in the urea product is 0.1 mole%. The remaining material fed to the first column exits through the top. This remaining material is fed to a second column where all water and urea in the column feed leaves through the column's bottom stream. The ammonia- rich stream leaving the second column's top stream is recycled back to a mixer where fresh ammonia and carbon dioxide are added. The effluent of this mixer is then fed to the reactor. A purge is installed on the recycle stream to remove the nitrogen from the system. This purge is operated so that 10% of the second column's top stream is purged. When operated at a fresh feed rate of 1,200 lbmole/hr of a 60 mole% NH3/38 mole% CO/2 mole % N mixture, the urea component flowrate leaving the first column's bottom stream is 321 lbmole NH4CO/hr. Determine the component molar flowrates (lbmole/hr) of all streams in the diagram on the following page as well as the overall and single-pass conversions of ammonia. Fresh NH3 N CO Mixer Reactor First Column N,H CO-Rich Stream Second Column Purge N NH, CO HO-Rich Stream
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


