Answered step by step
Verified Expert Solution
Question
1 Approved Answer
urgent! 2-1. DIAGRAM Ag-Sn 1000 800 - 72 600 Temperature/C 481 400 229 200 0.2 0.4 0.6 0.8 DATA 0.0 Ag 1.0 Sn Wa Q-1
urgent! 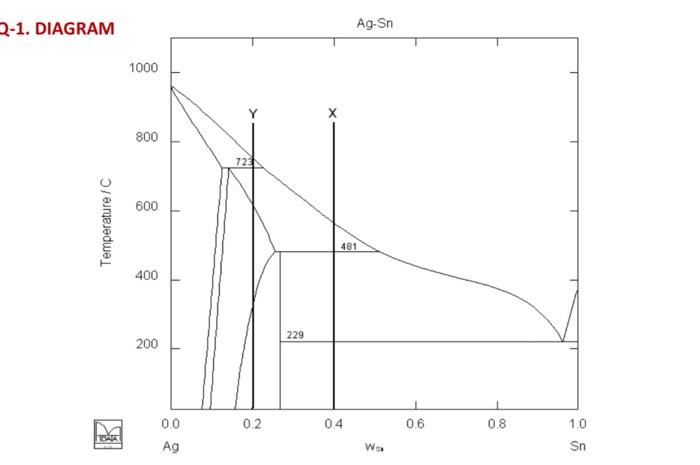

2-1. DIAGRAM Ag-Sn 1000 800 - 72 600 Temperature/C 481 400 229 200 0.2 0.4 0.6 0.8 DATA 0.0 Ag 1.0 Sn Wa Q-1 By using the Silver-Tin binary system given below, a) Label all areas in the diagram. (5 pt) b) Write all the reactions by indicating the reaction type. (10 pt) c) What is the solubility of Sn in Ag at 800C? (5 pt) d) Make a cooling study for composition Y (10 pt) e) Suppose 1 kg of alloy X is heated from room temperature to elevated temperatures. At what temperatures the first liquid phase will appear? How much liquid will be present at this temperature? (10 pt) f) During cooling, for composition Y, what is the amount liquid phase consumed during the reaction at 723C. (10 pt) Ag: 108 g/mol, Sn:119 g/mol 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


